The Pharmacist's Primer on Hematopoietic Stem Cell Transplants: Part 2
Steph’s Note: Ask and ye shall receive…part 2 of Dr. Rachel Hauf’s hematopoietic stem cell transplants. Considering Part 1 just came out a couple of weeks ago, we’re not making you wait long at all for this finale of the HSCT series! We don’t like to tease and leave you hanging super long like some other content creators (*cough* I’m looking at you, Stranger Things. Although let’s be honest, even waiting 24 hours would be too long to get season 5, so I guess what’s a year or 2…). Anyways, I digress. Let’s go, Part 2!
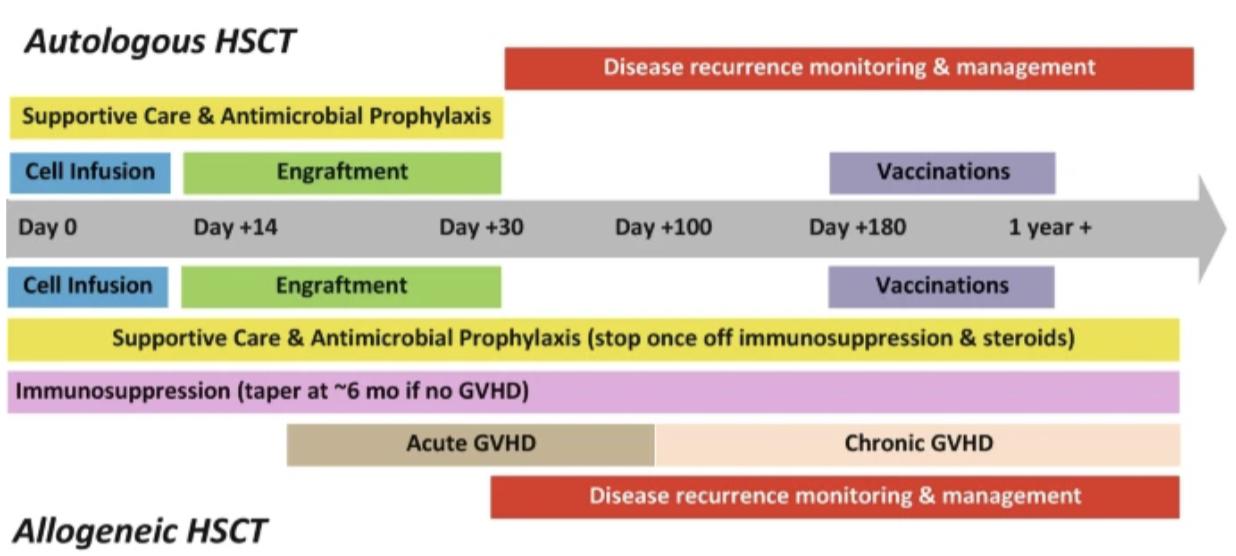
Now let’s talk about everything that happens AFTER a patient’s new birthday (aka their stem cell infusion). (Image)
Graft-versus-Host Disease (GVHD) and Management
Just when you think you have an ally in the donor cells, GVHD happens. Sigh. (Image)
Graft-versus-host disease (GVHD) may occur due to HLA mismatching or differences in minor histocompatibility antigens. The donor’s CD8 T lymphocytes register the recipient proteins as foreign and attack host tissue. (For a quick rundown on immune cells, check out this post!) There are two major types of GVHD: acute and chronic.
Historically, these subtypes have been differentiated by the timing of their occurrence. If GVHD occurred within 100 days of transplant, it was considered acute. If it occurred after 100 days, it was chronic. But these days, GVHD is classified based on presentation rather than timing.
Acute GVHD (aGVHD) primarily affects the skin, liver, and/or gastrointestinal (GI) tract. Skin manifestations include rash and bullae. Liver GVHD presents as jaundice and hyperbilirubinemia, and GI symptoms include diarrhea, nausea, and vomiting.
Grade 1 aGVHD is generally treated first-line with corticosteroids (methylprednisolone 2 mg/kg/day or prednisone 2-2.5 mg/kg/day). Lower doses of steroids are utilized for upper GI aGVHD. Topical steroids are preferred for skin involvement. For higher grades of GVHD, systemic corticosteroids, topical corticosteroids, and reinitiation or escalation of immunosuppressive agents may be required. Diarrhea may be controlled with oral budesonide or octreotide.
Chronic GVHD (cGVHD) may affect any organ. If any diagnostic feature according to the 2014 NIH Criteria for Diagnosing and Scoring Chronic GVHD is present, the diagnosis of cGVHD can be confirmed. Examples of such features include esophageal strictures, fasciitis, and lichen planus-like changes to various organs.
Management includes continuing, reinitiating, or increasing immunosuppressive agents, systemic corticosteroids, and topical steroids if needed. Pulmonary involvement can be treated with inhaled steroids, montelukast, and azithromycin. The ROCK2 inhibitor, belumosudil, is also useful in the treatment of cGVHD as it has the ability to prevent fibrotic tissue generation. Patients with steroid refractory GVHD may be treated with a variety of immunosuppressive agents, such as ruxolitinib, mycophenolate mofetil, calcineurin inhibitors, mTOR inhibitors, etanercept, and pentostatin.
It is worth noting that patients who have some GVHD have better responses than those who have none.
Wait, whaaaat?!
We just spent all this time talking about the sometimes awful manifestations of GVHD, and now we’re telling you we actually want to see some of this condition?? As weird as it may seem, yes, yes we do. It’s actually a signal that the donor cells are eliminating any residual disease (recall the graft-vs-malignancy effect).
GVHD Prophylaxis for Allogeneic HSCT
After allogeneic transplant, GVHD prophylaxis must be initiated to prevent the donor tissue from attacking the host. Such prophylaxis regimens commonly consist of a calcineurin inhibitor in combination with an antimetabolite or sirolimus. (If you’re trying to remember where you’ve read about these medications before with us, it was this post on solid organ transplant.) These medications are usually initiated at day -1 and generally may be slowly tapered at day +180 (6 months post-HCST) if no GVHD occurs. The optimal regimen depends on the relationship of the donor to the recipient, HLA matching, and the intensity of the conditioning regimen.
Calcineurin inhibitors inhibit the activation of T cells and are the backbone of GVHD prophylactic regimens. Cyclosporine (Gengraf) IV is initiated on day -1 at 3 mg/kg/day and then later converted to oral using an IV to PO conversion ratio between 1:2 to 1:4.
Tacrolimus (Prograf) has been demonstrated to be superior to cyclosporine in preventing high-grade GVHD. Similar to cyclosporine, it is initially given IV on day -1 at 0.03 mg/kg/day (ideal body weight) before conversion to the oral formulation. The conversion ratio per the package insert is 1:4 but may vary by institution.
Frequent trough monitoring is required to maintain a narrow target window of 8-12 ng/mL. When a patient has subtherapeutic levels, a pulse dose of steroids may be given to prevent GVHD. Tacrolimus is nephrotoxic. Renally impaired patients require more aggressive monitoring. It also decreases magnesium and potassium levels; therefore, magnesium oxide is started concurrently with tacrolimus. Management of diarrhea may be necessary secondary to magnesium supplementation. Monitor other medications that the patient is on that may decrease these electrolytes, such as diuretics.
The antimetabolites mycophenolate mofetil and methotrexate are often used in combination with calcineurin inhibitors. Mycophenolate mofetil (CellCept, MMF) decreases proliferation of T cells and reduces their response to antigens. It is administered at 10-15 mg/kg two to three times daily. Mycophenolate is generally discontinued at day +28 for all match-related transplants. For matched-unrelated transplant, it may be continued for 2 to 3 months post-transplant. IV methotrexate 15 mg/m2 on day +1 followed by 10 mg/m2 on days +3 and +6 (and sometimes +11) is standard.
Mycophenolate is associated with less toxicity and faster engraftment but has higher rates of more severe aGVHD. Like tacrolimus, mycophenolate requires supplemental magnesium. Mycophenolate mofetil is preferred for RIC or NMA regimens while methotrexate is preferred for MAC.
Sirolimus (Rapamune) is a mechanistic target of rapamycin (mTOR) inhibitor that prevents T cell activation and proliferation and increases T-regulatory cells, which are beneficial for shutting down alloreactive T cells. It is typically used in combination with tacrolimus. Sirolimus is initiated at day -3 at 2 mg once daily and adjusted to achieve a goal trough between 3 and 12 ng/mL. Some institutions may give a loading dose. Relevant adverse effects include thrombocytopenia, hyperlipidemia, and thrombotic thrombocytopenic purpura.
Posttransplant cyclophosphamide (PTCy) is a newer strategy for GVHD prevention due to its selectivity towards alloreactive donor T cells. Its efficacy in combination with tacrolimus and MMF was demonstrated in the BMT CTN 1703 study. Cyclophosphamide at a dose of 50 mg/kg is administered on days +3 and +4 after HSCT with mesna and IV fluids.
At first glance, it may seem puzzling to give chemotherapy after stem cell transplant. After all, wouldn’t it destroy the new cells we just infused?
Fortunately, PTCy is selective for alloreactive T cells while sparing T-regulatory cells and HSCs. These cells have high levels of aldehyde dehydrogenase, which make them resistant to cyclophosphamide. This is why we can give this drug post-HSCT. Historically, PTCy was used most frequently in HLA mismatched transplants, however, its utility has been expanded even to matched transplants.
Anti-thymocyte globulin (ATG) has the potential to reduce the incidence of chronic GVHD when added to calcineurin inhibitor-based regimens. There is mixed evidence regarding its impact on aGVHD. It is important to point out that the source of ATG is relevant. Rabbit ATG is used for GVHD. Thymoglobulin is the approved rabbit-derived ATG product in the US. ATG can result in severe hypersensitivity reactions, including anaphylaxis.
The addition of alemtuzumab (Lemtrada) 20 mg/kg to conditioning regimens has shown benefit in reducing rates of GVHD for unrelated donor transplants. It has a longer half-life than ATG and may therefore result in longer periods of lymphocyte depletion. Check out the Lemtrada REMS program here.
Abatacept (Orencia) is another IV agent that can be tacked on to a calcineurin inhibitor-based regimen for patients receiving a graft from an unrelated donor to reduce the incidence of aGVHD. 10 mg/kg is administered in days -1, +5, +14, and +28. Abatacept inhibits T-cell activation by binding to CD80 and CD86 on antigen presenting cells, thus blocking the co-stimulatory interaction with CD28 on T-cells.
Infection Prophylaxis Post-HSCT
(Image)
After transplant, patients are pancytopenic for prolonged periods and require prophylaxis against bacterial, viral, and fungal infection. Neutrophils start recovering first with a recovery time between 2 and 4 weeks, depending on the type of transplant. This is followed by platelet and red blood cell recovery, then finally B and T cells.
During periods of significant neutropenia, antimicrobial prophylaxis is required. In addition, G-CSF is administered post-HSCT to reduce the duration of neutropenia.
Infection prevention involves more than just drugs. Patients will need to make a lot of drastic temporary lifestyle changes. They should be encouraged to isolate, wear masks if they must be in public, and avoid sick people. Regarding diet, this population needs to avoid any form of uncooked meat (no rare steak, no sushi!), unpasteurized products, and runny eggs. Caution should be exercised with berries, too, which can harbor spores that stick even when washed. The list for neutropenic dietary recommendations goes on and on, so a dietician really should be consulted.
A sample of neutropenic precaution signage. Take note of how cautious these patients have to be…down to sending away any fresh flowers! (Image)
Antibiotics
From day 0 to +100, patients are at risk for infection with Gram negative bacilli, Gram positive bacteria, and Streptococci species that reside in the gut. After day +100, these patients are most vulnerable to encapsulated bacteria. Antibacterial prophylaxis with a fluoroquinolone should be considered for patients with neutropenia predicted to last at least 7 days. The fluoroquinolone should be initiated at the time of stem cell infusion and continued until recovery of the neutrophil count.
For patients who experience cGVHD or have low IgG levels, antibiotic prophylaxis against S. pneumoniae with oral penicillin should be started. Consider IVIG prophylaxis in patients with IgG <400 mg/dL. (For some background on IVIG, check out this post on NMOSD, in which IVIG is discussed.)
Antivirals
In the early post-transplant phase, patients have an increased risk of herpes simplex virus (HSV) infection. Patients who are seropositive should receive prophylaxis with acyclovir to prevent reactivation. Prophylaxis should begin at the start of conditioning and continue until around day +30.
For patients who are varicella zoster virus (VZV) positive, acyclovir is continued for the first year after transplant.
Cytomegalovirus (CMV) prophylaxis is started on the day of transplant and continued to day +100. At this time, the immune cells are competent enough to prevent CMV reactivation. Most of the population is actually infected with CMV latently; if either the donor or recipient is positive for CMV, the recipient must receive prophylaxis. CMV infection in a patient post-transplant can lead to graft loss.
Letermovir (Prevymis) is a commonly used prophylactic agent that targets CMV DNA terminase, preventing viral replication. Ganciclovir, acyclovir, and valacyclovir are alternative agents that can be considered. Letermovir may be preferred as it does not cause cytopenias. Furthermore, even when letermovir is discontinued, the benefit persists after 2 years.
Antifungals
Antifungal prophylaxis is traditionally only given to allogeneic transplant recipients. Candida and Aspergillus species pose the biggest threat directly after transplant takes place. Prophylaxis is initiated during conditioning, and the duration is determined on an individualized basis.
Fluconazole is generally preferred, and alternatives include itraconazole, posaconazole, voriconazole, and micafungin. Be mindful of drug-drug interactions as the azoles are moderate to strong CYP3A4 inhibitors. One notable interaction is with calcineurin inhibitors used for GVHD.
Pneumocystis jirovecii is another fungal infection commonly seen in transplant patients, necessitating prophylaxis until at least 6 months post-transplant. Prophylactic options include trimethoprim-sulfamethoxazole (Bactrim), pentamidine, dapsone, or atovaquone. Although Bactrim has superior efficacy, it is also myelosuppressive. Dapsone should be avoided in patients with G6PD deficiency.
When allogeneic HSCT patients are most susceptible to which infections. Now before you go and memorize each time period, don’t forget that patients who continue to receive prolonged immunosuppression may require longer periods of antimicrobial prophylaxis. So it’s still individualized. (Image)
Post-HSCT Immunizations
Allogeneic stem cell transplant recipients lose all immunity from previous vaccinations due to the harsh conditioning regimens. Their immune systems are basically akin to that of a newborns’, making revaccination imperative. The exact scheduling of vaccinations may vary from institution to institution, but here are some general guidelines.
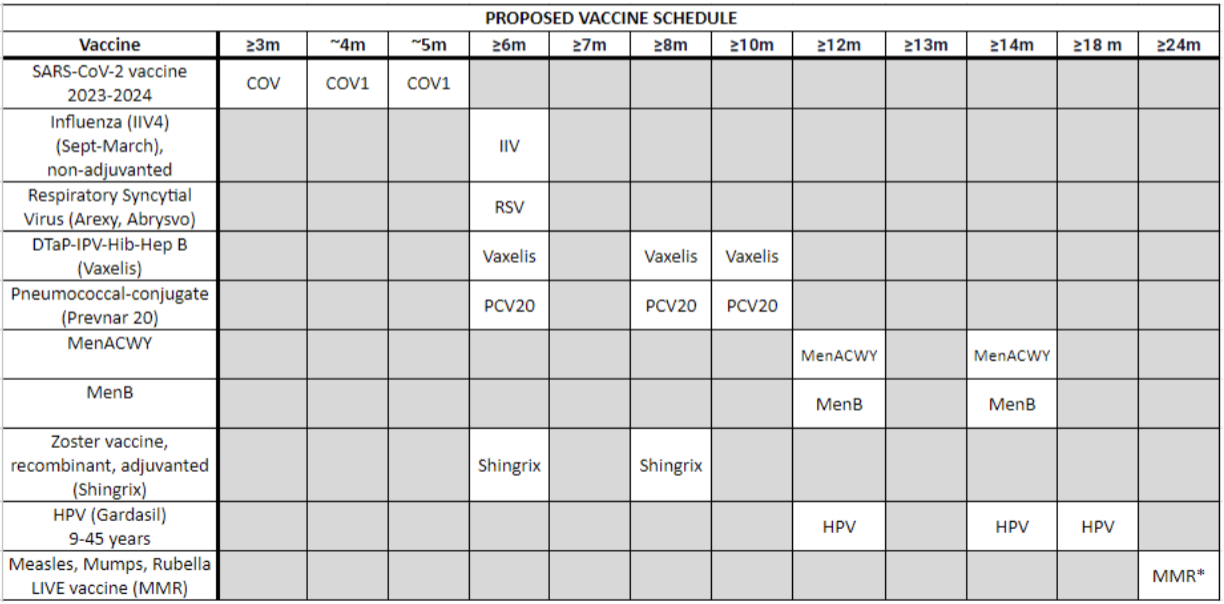
The influenza vaccine (IIV) may be given annually starting 6 months after transplant; it may be given as early as 3 months post-HSCT during peak flu season. The 3-dose SARS-CoV-2 vaccine series starts at +3 to 6 months. Generally, the three-dose pneumococcal vaccine series is administered 3 to 6 months post-transplant at 4-week intervals. The H. influenzae type B vaccine series also consists of three doses spaced 1 month apart, starting 6 months after HSCT.
The Diptheria, tetanus, and pertussis (DTAP) vaccine is typically started 6 months post-transplant and consists of 3 doses spaced 1-2 months apart. The poliovirus vaccine (IPV) is another 3-dose series given 1-2 months apart starting at +6 to 12 months. The 3-dose hepatitis B vaccine (HBV) series is given at months 6, 8, and 12. The Neisseria meningitidis vaccines, MCV-4 and MenB, are both started 6 months post-HSCT and consist of 2 doses given at 2-month intervals.
The herpes zoster virus vaccine (Shingrix) is often given at months 6 and 8. The HPV and Hepatitis A vaccines may also be considered for specific patient populations.
Even with variability between institutions, the BIGGEST thing to remember here is this: live vaccines (LAVV, MMR) should be avoided until at least 24 months post-transplant.
Here’s a sample post-transplant immunization schedule:
Childhood vaccines all over again, just a little sped up in some cases.
Donor Lymphocyte Infusions (DLIs)
DLIs may be utilized to eliminate minimal residual disease or prevent relapse in allogeneic stem cell recipients by boosting the graft-vs-malignancy effect. Essentially, we are simply giving more donor stem cells to the patient. The infusion also contains donor lymphocytes as well. These donor T cells help the donor stem cells survive the hostile environment of the new host.
Autologous vs Allogeneic Transplant Recap (The tl;dr)
What did I tell you? Stem cell transplant is a universe of its own, but I hope these articles have made it at least somewhat less of a mystery. We discussed a lot, so I compiled a summary table of the basics you should be aware of in true tl;dr fashion: