The Pharmacist's Primer on Hematopoietic Stem Cell Transplants: Part 1
Steph’s Note: This week, we’re taking a turn from our recent critical care forays to delve into one of the most fascinating (and complicated) oncology topics - stem cell transplants. (Yes, we’ve written about this topic previously, but this time, we’re armed with MORE drug info.) Because it’s such a dense topic, we’re breaking it up into 2 parts to allow you time to fully digest. FYI, stem cell transplants are not only utilized in the oncology world. They’re also gaining traction as a treatment for non-malignant conditions like multiple sclerosis, primary immunodeficiency disorders, hemoglobinopathies like sickle cell disease, and the list goes on. So even if you have zero (or subzero) interest in oncology, you just might encounter patients in other realms who have had an HSCT.
Here to break down this complex topic is Rachel Hauf, PharmD. Rachel graduated from Texas Tech University’s School of Pharmacy in May 2023 and is currently completing NCODA's Medically-Integrated Oncology Pharmacy (MIOP) residency program at Texas Oncology in the Dallas-Fort Worth metroplex. She previously has shared her experiences and advice for pursuing a residency program as well as her knowledge of pharmacy financial metrics. Time to drop (some knowledge) like it’s hot, Rachel!
Rachel’s Note: Special thanks to my BMT preceptor and Texas Oncology’s stem cell transplant pharmacist, David Samuel, PharmD, who turned such an intimidating topic into an exciting challenge. I was fortunate to learn from a mentor with such incredible mastery of the subject and an equally admirable dedication to the transplant patient population.
Introduction to Hematopoietic Stem Cell Transplants
The world – or rather, the universe – of stem cell transplant is fascinating. Referring to transplant as simply a “world” is an understatement of all it encompasses. Yes, “universe” is much more suitable. These transplants require extensive planning, intensive chemotherapy, and rigorous supportive care.
Before I get in too deep, I do want to reiterate that this is an advanced topic. There are certain foundational concepts you need to be familiar with to fully understand this article. If you need to, go ahead and brush up on T-cell structure/function, G-CSFs, and HLA matching. (This tl;dr post on the immune system is a good start for at least some of this info!)
Hematopoietic stem cell transplant (HSCT) involves collection of stem cells from either the patient or a donor with subsequent infusion of those cells into the patient after chemotherapy. There are two types of HSCT: autologous and allogeneic. An autologous transplant involves reinfusion of the patient’s own stem cells, while an allogeneic transplant involves infusion of donor cells. The choice of transplant type depends on the clinical scenario.
Sometimes the immune system does stuff that we just plain don’t understand. Ask anyone with a peanut allergy. Or raging seasonal allergies.
Prior to transplant, an intense conditioning chemotherapy regimen is administered. Transplant patients are extremely immunosuppressed due to conditioning as well as due to their post-transplant anti-rejection medications. They therefore require extensive supportive care with infection prophylaxis. In addition, after transplant, a patient’s immune system is rendered essentially equivalent to that of a newborns’, necessitating revaccination from scratch.
The transplant timeline is complex, and there is certain terminology that must be understood before we proceed. Day “0” refers to the date that the patient actually receives the transplant. (Some centers call this the patient’s new birthday :)) Days with a negative symbol (day -2, -1, etc) refer to the days preceding the transplant. Days with a positive symbol (day +1, +2, etc) refer to the days following the transplant.
Now that we have some of those basics out of the way, buckle up, we’ve got a lot to cover.
Stem Cell Collection and Mobilization
There are three sources of stem cell collection:
the bone marrow,
the peripheral blood, or
umbilical cord blood (UCB).
Peripheral blood stem cell (PBSC) collection may be performed for either autologous or allogeneic transplants. This is accomplished through a process called apheresis, which involves filtering the blood for HSCs. The HSCs are separated out and the remaining blood product is reinfused back into the patient (think of it as a process similar to dialysis). PBSC collection is considered the easiest method of collection and is associated with rapid recovery of blood counts. However, it is associated with higher rates of graft-versus-host disease (GVHD) compared to bone marrow.
To make this totally clear, that long needle has to go through the bone into the marrow to collect the cells. It’s not always a pretty thing. (Image)
While only PBSCs are used for autologous transplants, PBSC, bone marrow, and UCB can all be considered as sources of collection for allogeneic transplant. Collection from the bone marrow is more invasive and requires local or general anesthesia. The marrow is aspirated from the posterior iliac crests using an intimidatingly long needle with a T-shaped handle.
I had the…pleasure…of observing this procedure while on my transplant rotation (the procedure went well for the patient, but not so much for me!). Unfortunately, this source of HSCs is associated with a higher incidence of graft failure.
Parents may choose to donate their baby’s umbilical cord blood (UCB) to a public cord blood bank. UCB contains HSCs that can be used for transplant, albeit resulting in smaller quantities of HSCs compared to the other sources of stem cells. Adult transplant recipients often require two UCB units. Although UCB can be obtained quickly, it is associated with slower recovery of blood counts.
In this rather (ahem, very) simplified overview of the stem cell differentiation, you’ll see that CD34+ stem cells can become any member of the hematopoietic cell families. This is why they are used to reconstitute patients after conditioning regimens. (Image)
As you can see, there are advantages and disadvantages to consider with each mode of collection.
So, we all know that HSCs reside in the bone marrow. How can we harvest HSCs from the peripheral blood, then? I’m glad you asked!
All pluripotent stem cells express CD34 early in development. These pluripotent cells are the ones we are looking to collect. CD34+ stem cells are mobilized out of the marrow and into the peripheral blood using granulocyte-colony stimulating factor (G-CSF). Administration of G-CSF increases the proliferation of HSCs. Patients generally receive 4 doses of G-CSF at 10 mcg/kg/day for 4 days, and PBSC collection then proceeds.
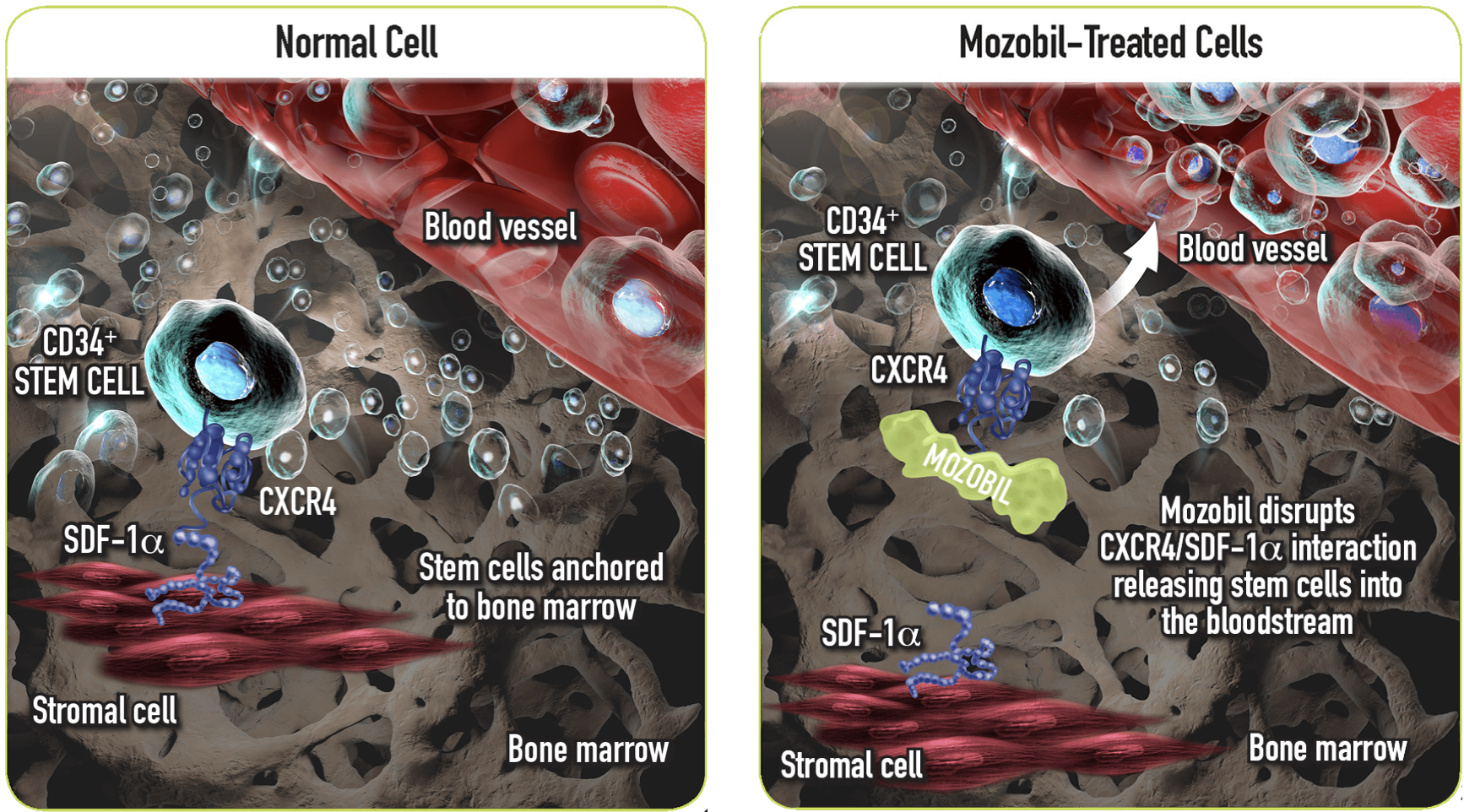
If enough cells cannot be collected initially, plerixafor is generally administered. Plerixafor (Mozobil) is a CXC chemokine receptor 4 (CXCR4) inhibitor, facilitating the mobilization of stem cells from the bone marrow into the blood. Motixafortide (Aphexda) is a newer stem cell mobilizing agent that was approved in 2023. It has a similar mechanism of action as plerixafor but is longer acting.
Mozobil = plerixafor. You can think of the CXCR4 and SDF-1a interaction as a tether for the CD34+ stem cell to stay in the bone marrow. Plerixafor interferes with this anchoring mechanism, allowing the stem cell to leave the marrow. Let’s have a moment for the scientific brilliance of the people developing these medications! (Image)
Some chemotherapy agents can also be used to mobilize stem cells, with regimens most commonly containing cyclophosphamide or ifosfamide.
The ideal amount of stem cells needed for transplant varies by institution but is generally in the ballpark of 5 to 6 million cells/kg. The goal is to collect enough cells to have a surplus that can be stored and potentially used for a second transplant, if needed. There’s a balance between giving too many or too few stem cells. Administer too many, and graft-vs-host disease will be a massive issue. On the other hand, administer too few, and the graft may fail. Engraftment occurs when the transplanted stem cells begin to proliferate in the recipient’s bone marrow.
Conditioning Regimens Prior to Stem Cell Transplant
The purpose of conditioning regimens is two-fold. First, they need to suppress the immune system so that it will accept the donor graft. Second, they need to eradicate the cancer cells. (For autologous transplants, only the latter is relevant.) Conditioning regimens fall into 1 of 3 categories:
myeloablative,
reduced intensity, or
nonmyeloablative.
Myeloablative conditioning (MAC) regimens result in significant and prolonged pancytopenia. Examples of MAC regimens include busulfan/cyclophosphamide (Bu/Cy), melphalan, total body irradiation (TBI)/cyclophosphamide, fludarabine/busulfan (Flu/Bu), and BEAM. MAC is preferentially utilized for autologous transplant and for allogeneic transplant in those who are younger and otherwise healthy.
Reduced intensity conditioning (RIC) utilizes lower doses of cytotoxic conditioning drugs, resulting in less toxicity. However, it doesn’t completely eradicate the cancer before transplant. In this instance, we rely on the graft-versus-malignancy effect for the clearance of any remaining malignant cells. (FYI this is different than graft-versus-host disease.) The graft-versus-malignancy effect is facilitated via donor alloreactive T-cells. (However, when these alloreactive T-cells target non-hematopoietic tissue, graft-versus-host disease occurs- we’ll discuss this in Part 2).
RIC is often used for multimorbid or elderly patients, but it has been increasingly used in more fit patients as well. The downside to these RIC regimens is that they are associated with a higher risk of relapse. Examples of RIC include fludarabine/melphalan (Flu/Mel), Flu/Bu (less intensive dosing than MAC), fludarabine/cyclophosphamide (Flu/Cy) with or without TBI and thiotepa.
Nonmyeloblative (NMA) regimens are even less intensive than RIC. We generally only consider these regimens for patients with a low burden of disease, and again, we are really banking on that graft-versus-malignancy effect to come through. Examples of such regimens include Flu/TBI and total lymphoid irradiation/antithymocyte globulin (TLI/ATG).
Check out these examples of conditioning regimens:
Alright, based on this image alone, raise your hand if you think pharmacists are necessary for HSCTs… This is just an example of pediatric regimens and doses, but you can see how complex conditioning regimens can be! Then add in the correct dosing, adverse reactions, monitoring, drug interactions, oh my! (Image)
The choice of conditioning regimen and dosing differs for autologous and allogeneic transplant, as well as by disease state. Keep in mind that some conditioning drugs require dose adjustments for obesity, poor performance status, age, and renal impairment.
These conditioning regimens can result in a multitude of complications. Hepatic sinusoidal obstruction syndrome (SOS), aka hepatic vein occlusion (VOD), is a rare complication of HSCT that is associated with high mortality. This toxicity is more common with allogeneic transplant and generally arises within the first month post-HSCT, but it can occur up to several months afterwards. Ursodiol is an oral liver protectant that decreases hydrophobic bile acids, which are toxic to hepatic cells. It is started on day -1 at 12 mg/kg/day in two divided doses and usually continued until day +90.
Dry mouth is a common adverse effect related to conditioning and can lead to gingivitis or even thrush if not managed appropriately. Patients should be encouraged to brush teeth regularly, floss, and rinse their mouth frequently. Palifermin (Kepivance) may be used for oral mucositis associated with autologous HSCT conditioning regimens.
Confused by all the acronyms yet? I hope not, because there are many more to come!
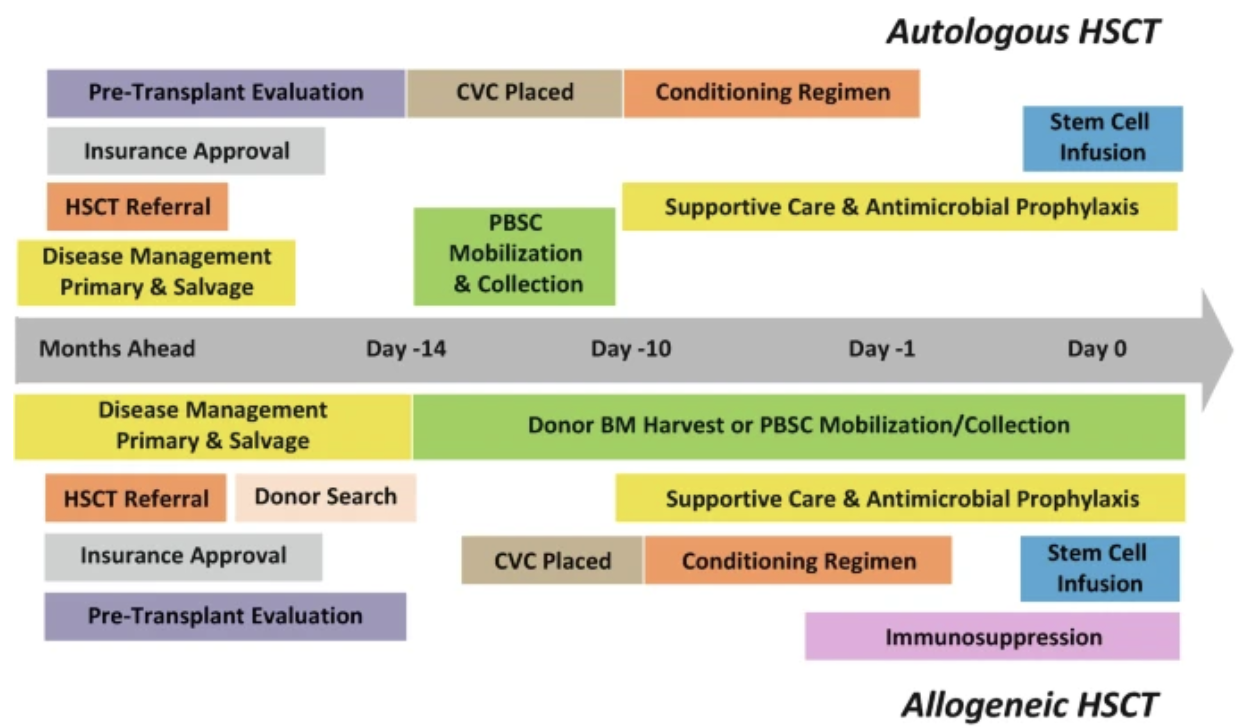
Pre-HSCT Timeline
Just take a moment to think about how many people and disciplines are involved in the pre-HSCT process. We’re talking physicians, financial/access staff, stem cell/match/bank specialists, line/access providers, pharmacists, nurses, social workers to prepare patients mentally, etc. It’s also coordinating not one patient’s schedule but TWO, if it’s an allogeneic transplant. This is HUGE. And it’s just the beginning. (Image)
Autologous Transplant
Generally, myeloablative conditioning regimens are utilized in the setting of autologous transplant. Remember, in autologous transplant, the patient is just getting their OWN cells back. Therefore, a myeloablative regimen is required to effectively wipe out the malignant cells since there will be no graft-vs-malignancy effect to rely on in this scenario.
After the administration of myeloablative conditioning, the patient’s HSCs are reinfused to replace those that were eradicated during conditioning.
Allogeneic Transplant
A stem cell transplant donor must match the recipient in terms of specific major histocompatibility complex (MHC) genes. These are referred to as human leukocyte antigen (HLA) genes in humans, and HSCTs particularly focus on HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1.
(Image)
Histocompatibility refers to the possession of similar alleles of a specific set of HLA genes. These genes code for the production of MHC class I and II proteins. If MHC proteins between donor and recipient aren’t similar enough, the donor’s T cells will identify the recipient’s MHC proteins as “foreign” and launch an attack. Thus, a highly matched donor/recipient pair increases the chance of successful engraftment and decreases the risk of graft-versus-host disease (GVHD).
The ideal donor is an identically HLA-matched sibling. The next best thing would be an unrelated, completely matched donor. After that, either a haploidentical related donor, who shares at least one HLA haplotype with the recipient, or UCB could be considered (a haplotype is one complete set of HLA-A, -B, -C, -DRB1, and -DQB1 alleles).
And this is where Steph shamelessly plugs for the Be the Match program, now known as NMDP. Her dad had a matched unrelated donor from the registry for his allogeneic HSCT in 2008 since there were no familial matches. Register now if you haven’t already… You could save a life!
The tl;dr of Hematopoietic Stem Cell Transplants (Pre-Transplant)
Don’t worry. If this feels like a bit of a cliff hanger ending, you’re right. It is. But now that we’ve covered the terminology and basics of pre-HSCT, you’ll be ready to cover everything for post-HSCT next - including the beast of GVHD. Stay tuned for the “post” post soon!
To summarize:
HSCTs are used to treat a variety of oncologic and non-oncologic conditions due to their ability to “reset” the immune system using pluripotent stem cells.
Depending on the condition being treated, the HSCT will either be autologous or allogeneic.
Stem cell collection sources and conditioning regimens vary widely depending on individual patient characteristics and the disease state being treated. Pharmacotherapy is integral to both steps to ensure adequate stem cell supply for the transplant and optimal host environment for engraftment.
Pharmacists play a HUGE role in HSCTs given the complicated medication regimens for which timing can be key, as well as the potential for drug interactions and adverse reactions. Multi-disciplinary teamwork is imperative!