The Pharmacist's Guide to Initial Resuscitation for Sepsis & Septic Shock
Steph’s Note: If you’re wondering why the title of this post doesn’t simply stop after “Sepsis,” this is the article for you. (Btw, I also would’ve thought the same thing in pharmacy school, so you’re not alone!) Here this week to explain why we all would’ve been missing a crucial bit of the sepsis story is Dr. Josef Nissan. If you don’t know Joe already from posts like this one on toxicology or from several of our handy pocket guides, you probably sense a hole in your pharmacy heart. Fill it in starting now.
I remember going through the majority of my APPE rotations using the terms sepsis and septic shock interchangeably. Then I got to my ID rotation, and my preceptor asked, “What’s the difference between sepsis and septic shock?”
We’ve hired Stanley to assist us with cheat sheet sales. We’re expecting him to be super effective, no? (Image)
You can say I struggled answering that question. But because I care so deeply about our tl;dr audience, I want you to avoid making the same mistake that I did. So strap in because we’re going for a ride on the sepsis train.
P.S. (shameless plug)! If you want a condensed cheat sheet of everything that we will go over today and more, take a look at our tl;dr sepsis cheat sheet. It has everything you need to know including pathophysiology, diagnosis, one-hour bundle, antibiotic clinical pearls, crystalloid fluid resuscitation, vasopressors, and so much more!
Sepsis Versus Septic Shock
Alright let’s start with the basics. So what’s the difference between sepsis and septic shock? Pretty much, one is the precursor of the other. More specifically:
Sepsis: Dysregulated systemic inflammatory and immune response to microbial infection that produces organ injury
Mortality Rate: 15-25%
Septic Shock: Sepsis with hyperlactatemia and concurrent hypotension requiring vasopressor therapy
Mortality Rate: 30-50%
Pathophysiology of Sepsis and Septic Shock
Sepsis has a very complex biochemical pathophysiology. However, this is tl;dr and let’s be real, we all want a condensed and simplified version of it. So let’s make it as basic as possible.
(Image)
For one reason or another, bacteria find their way through an organ and into the bloodstream (generally due to an untreated infection such as a UTI, pneumonia, skin & soft tissue infection, etc). And as you probably know, bacteria don’t belong in our bloodstream (duhh?).
Therefore, our body freaks out and activates numerous different pro-inflammatory and anti-inflammatory markers including cytokines, proteases, kinins, reactive oxygen species, and nitric oxide, leading to vasodilation, capillary leakage, and intravascular volume depletion. If left untreated, this can lead to severe hypotension, organ failure, and ultimately death. This cascade of events is responsible for the clinical signs and symptoms of sepsis and progression to septic shock.
Sepsis Screening Tools
Unfortunately, there is no single diagnostic test for sepsis. It is generally diagnosed using a combination of different lab tests, microbial cultures, and clinical judgment. That being said, there are two major screening tools that can be used to help identify patients with suspected infection who are at greater risk for poor outcomes. Take a look below:
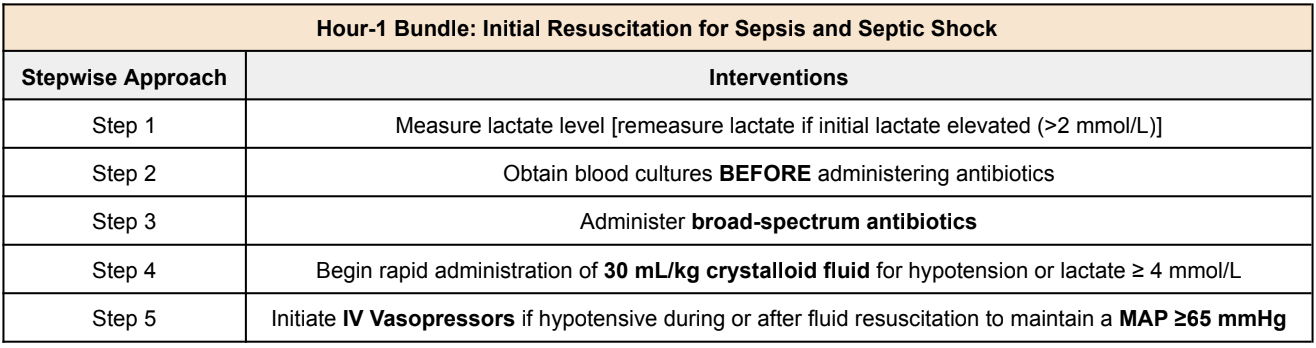
What is the One-Hour Sepsis Bundle?
So what the heck is this one-hour sepsis bundle that I keep referring to? Well, as you have seen, sepsis is a life-threatening condition with very high mortality when not treated in a timely manner. So to make our lives easier, the Society of Critical Care Medicine (SCCM) created a one-hour bundle to help with the initial resuscitation phase for sepsis and septic shock. Here’s a brief overview of everything that we’ll talk about.
One-Hour Sepsis Bundle - Step 1 (Lactate)
So what on earth is lactate, and why is it the first step in the initial resuscitation of sepsis? Oh I am so glad you asked.
Lactate is a byproduct that is released by our organs (e.g., skeletal muscle, brain tissue, GI tract, etc) during anaerobic metabolism. So why is that helpful in diagnosing sepsis? Think about it. It helps give us an idea of organ perfusion. Still confused? Ok, let’s simplify.
Arnold does not have an issue with tissue perfusion here. (Image)
I enjoy weight lifting. So let’s use my bicep in this example. As I am sitting here typing, I am currently sepsis free. Meaning I have no bacteria in my blood and therefore no anti-inflammatory and pro-inflammatory markers are being released. So my blood pressure is currently stable, and I do not have excess vasodilation. My bicep is getting adequate oxygen from my red blood cells and is therefore undergoing aerobic metabolism.
Ok let’s flip the script.
Let’s say I now have untreated E. coli bacteremia. My body freaks out, releases a bunch of cytokines and nitric oxide, leading to severe vasodilation and capillary leakage. This severe fluid loss and vasodilation are going to cause my blood pressure to decrease. As my blood pressure continues to drop, less blood flow is going to my bicep, leading to hypoperfusion. This forces my bicep to undergo anaerobic metabolism due to the lack of oxygenation. During anaerobic metabolism, my bicep will release the byproduct lactate. Make sense now?
So as you can see, higher lactate means decreased tissue perfusion. Aka, more severe sepsis/septic shock.
One-Hour Sepsis Bundle - Step 2 (Blood Cultures)
^^How it feels when the blood cultures are drawn after antibiotics are given… :/ (Image)
Not too much to talk about here, honestly. Pretty self explanatory. With sepsis, we are concerned for bacteremia. So how can we test for it? Obtain blood cultures of course. The only important thing to remember here is ALWAYS collect blood cultures BEFORE administering antibiotics. Why? Because if you give antibiotics before, there is a chance it may eradicate the bacteria leading to false negative blood cultures.
Simple enough. Next step.
One-Hour Sepsis Bundle - Step 3 (Broad-Spectrum Antibiotics)
So how do we actually treat sepsis? By getting rid of the bacteria in the blood. And how do we do that? With antibiotics of course.
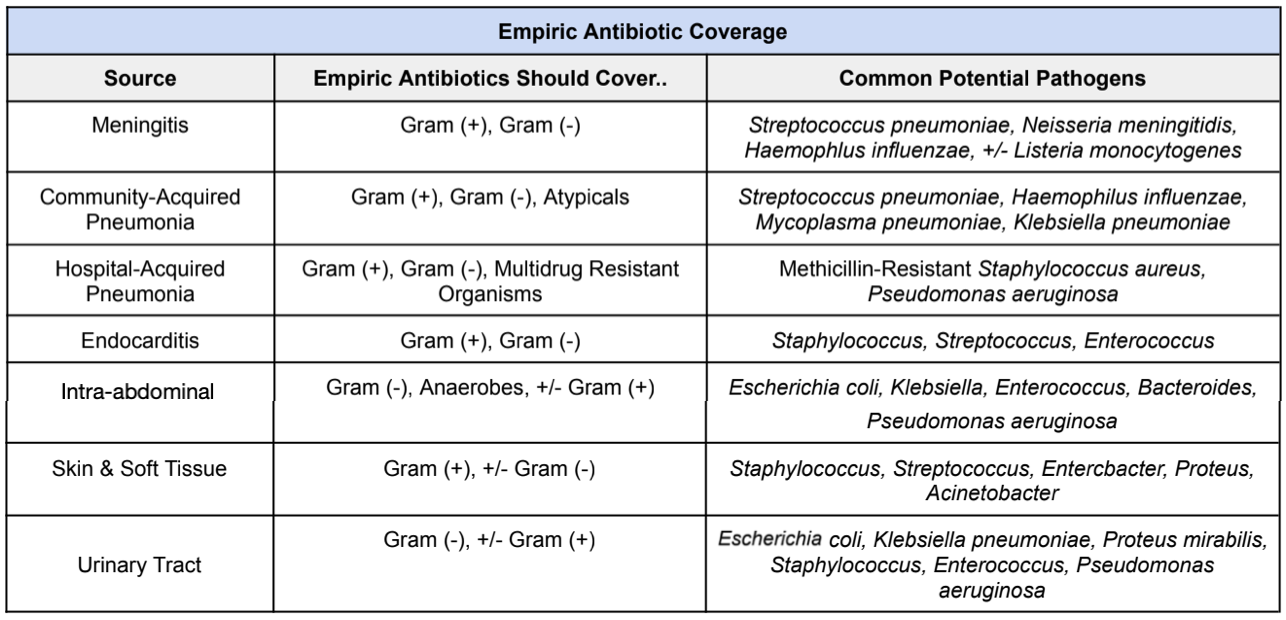
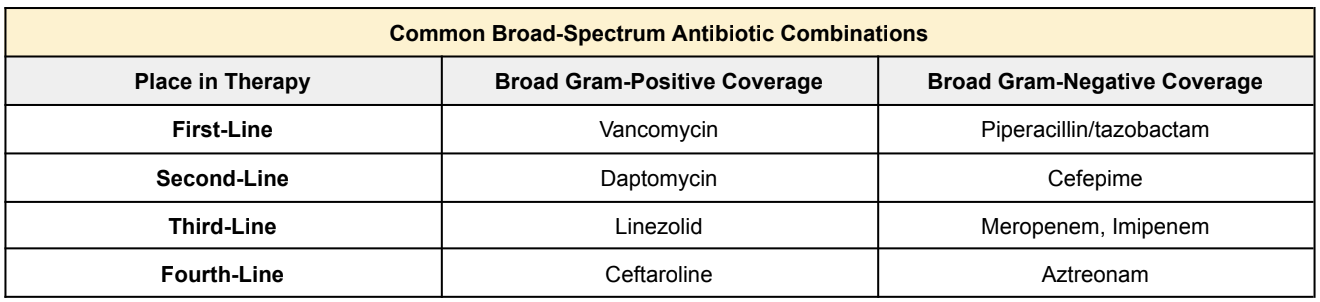
Now let me start by throwing a big disclaimer at you. Each institution may differ between first-line antibiotic selection. Unfortunately there’s no standardized antibiotic selection for all patients with sepsis. There are so many different factors that come into play when selecting the best broad-spectrum antibiotic coverage. For example, everyone knows vancomycin & Zosyn (piperacillin/tazobactam), right?
But let’s use our clinical judgment here. Does someone coming in for urosepsis without a chronic foley catheter really need to be empirically started on vancomycin? Probably not, right? So always make sure to use your clinical judgment to assess the likely source to help you figure out which empiric antibiotics should be started. A good rule of thumb to follow is:
So now that we have an idea of which pathogens to empirically cover for depending on the source of infection, let’s talk about some common broad-spectrum antibiotic agents that are often used in the initial treatment of sepsis.
Again, big disclaimer here!!! Each institution may differ between first-line agent selection. It may be clinically reasonable to start antibiotics NOT listed below.
One-Hour Sepsis Bundle - Step 4 (Crystalloid Fluid)
As you may remember, when we discussed the pathophysiology of sepsis, we talked about the cytokine release leading to vasodilation and capillary leakage. During capillary leakage, intravascular fluid leaks extravascularly, leading to intravascular volume depletion. Severe capillary leakage can lead to hypovolemic shock and tissue hypoperfusion. So how can we treat this intravascular volume depletion?
By giving crystalloid fluid of course!
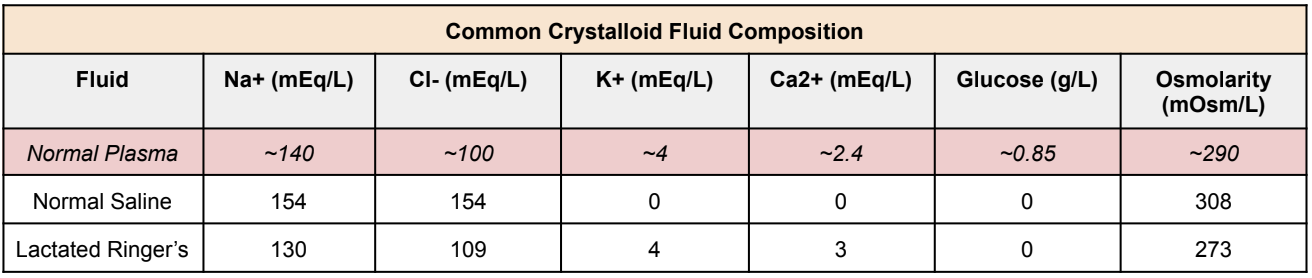
How much fluid should we give? 30 mL/kg of IV crystalloid fluid.
Now again, clinical judgment should be used. In septic patients with a history of CKD and heart failure, you may not need as much fluid repletion. So make sure you always assess each patient before starting fluids. That being said, the two most common IV crystalloid fluids that are used are normal saline and lactated Ringers. What’s the composition difference between the two fluids? Take a look below:
One-Hour Sepsis Bundle - Step 5 (Vasopressors)
Ah, my favorite part :). Again, this all comes back to the pathophysiology of sepsis. Bacteria in the blood → cytokine & nitric oxide release → vasodilation & capillary leakage → hypotension → tissue hypoperfusion → death. You got it? Ok, good.
So, when do we start vasopressors? ONLY if the patient remains hypotensive during or after fluid resuscitation to maintain a MAP ≥ 65 mmHg. Why do we aim for a MAP greater than 65 mmHg?
It’s not random. The most important organ in the body is the brain (you can argue with me though). So a MAP of 65 mmHg is the minimum vascular pressure that is needed to continue to perfuse the brain. Anything less than that leads to brain hypoxia and death. So now you know why we aim for this seemingly arbitrary number.
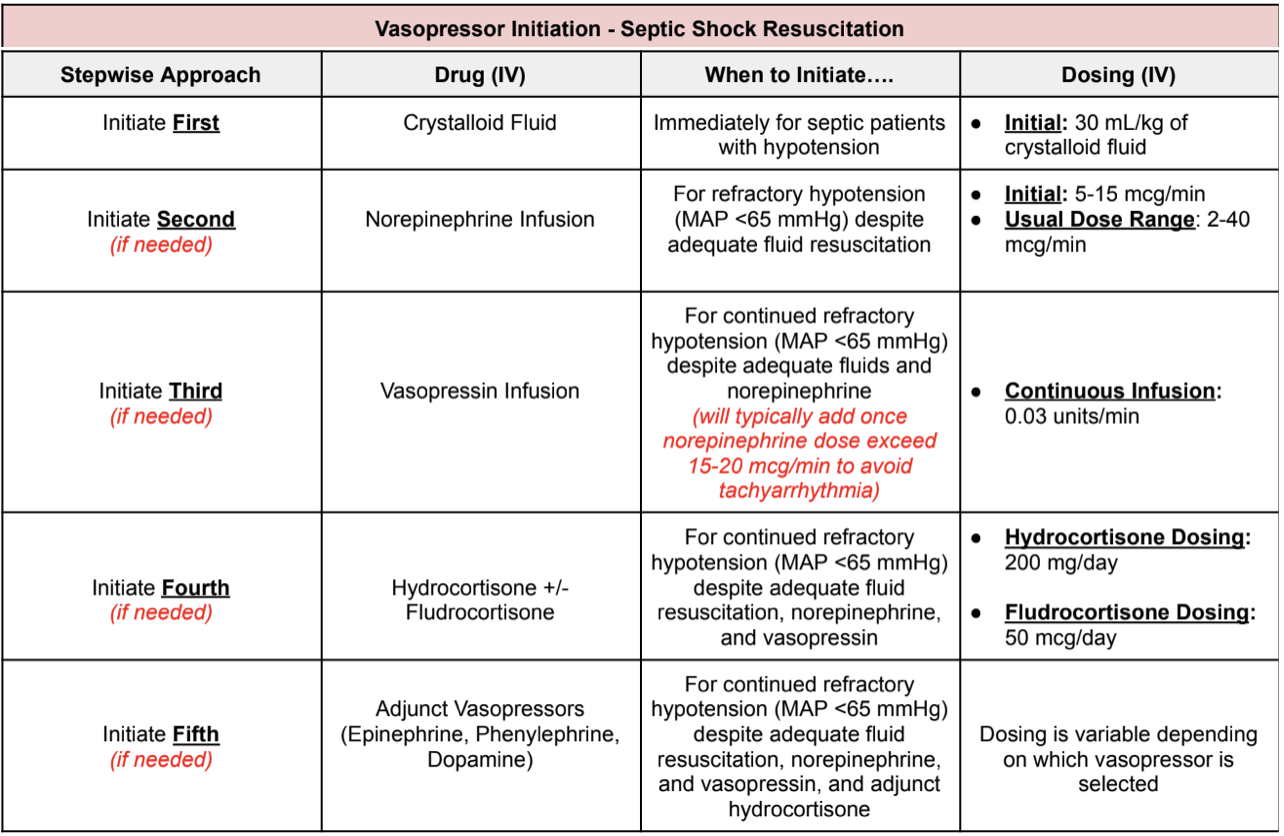
Ok, so which pressors do we start? Well, I made a table for you that makes it pretty easy to follow. Take a look:
Now we know when and which vasopressors to start for patients with refractory septic shock. Let’s say you did a miraculous job and the patient’s blood pressure has improved immensely. So how do we peel back on vasopressors? Go backwards of course.
For example let’s say you have patient JJ who came in for septic shock secondary to CAP. JJ was given IV fluids and was started on norepinephrine, vasopressin, hydrocortisone, and phenylephrine. If MAP has continued to improve, start by taking off phenylephrine → then hydrocortisone → then vasopressin → then norepinephrine. Pretty simple, right?
The tl;dr of Sepsis & Septic Shock
Sepsis and septic shock are not the same thing. Untreated sepsis can lead to septic shock.
Sepsis: Dysregulated systemic inflammatory and immune response to microbial infection that produces organ injury
Septic Shock: Sepsis with hyperlactatemia and concurrent hypotension requiring vasopressor therapy
Bacteremia leads to the activation of numerous different pro-inflammatory and anti-inflammatory markers including cytokines, proteases, kinins, reactive oxygen species, and nitric oxide leading to vasodilation, capillary leakage, and intravascular fluid depletion. If untreated, this can lead to severe hypotension, organ failure, and ultimately death. This cascade of events is responsible for the clinical signs and symptoms of sepsis and progression to septic shock.
There is no single diagnostic test for sepsis. It is generally diagnosed using a combination of different lab tests, microbial cultures, and clinical judgment. Common diagnostic screenings to help diagnose sepsis include the qSOFA & SIRS screening.
The Society of Critical Care Medicine recommends the following one-hour bundle for the initial resuscitation of sepsis/septic shock:
Step 1: Measure lactate level
Lactate is a byproduct released during anaerobic metabolism. Increase in lactate means increase in tissue hypoperfusion
Step 2: Obtain blood cultures BEFORE administering antibiotics
Step 3: Administer broad-spectrum antibiotics
Always use clinical judgment when starting broad-spectrum antibiotics
Step 4: Begin rapid administration of 30 mL/kg of crystalloid fluid for hypotension or lactate ≥ 4 mmol/L
A goal MAP of 65 mmHg is recommended to ensure appropriate brain perfusion
Patients with obvious hypovolemia should be resuscitated with 30 mL/kg of IV crystalloid therapy
Step 5: Initiate IV vasopressors if hypotensive during or after fluid resuscitation to maintain a MAP ≥65 mmHg
Always use the following algorithm when starting vasopressors for the treatment of septic shock:
Initiate first: crystalloid fluids
Initiate second: norepinephrine
Initiate third: vasopressin
Initiate fourth: hydrocortisone +/- fludrocortisone
Initiate fifth: other adjunctive pressers, including phenylephrine, epinephrine, dopamine, and angiotensin II