Using Direct Oral Anticoagulants in Special Populations
Steph’s Note: After a few month’s break from anticoagulation, we’re back! You know we love us some blood thinners on this site (really a reflection of how integral these medications are to daily practice). And I have to say, anticoagulants are back with a BANG. We have a monster post here from two dedicated PharmDs-to-be (very soon!), and they have outdone themselves. You should probably just send them fan mail now.
Suman Augsteen is a P4 student at the University of Texas at Austin College of Pharmacy. She is passionate about direct patient care and hopes to practice in the community health center setting after graduation. When she’s not at rotations learning as much as she can, she enjoys cooking, running, and trying to find all the cute coffee shops in Dallas.
Carmela Noche is a P4 student at the University of Texas at Austin College of Pharmacy. She is excited about outpatient care and advocating for underserved communities. Outside of pharmacy, you can find her eating ice cream or exploring a new city.
Suman and Carmela, teach us!
Imagine yourself as a P-something sitting in lecture in the very front back* row, learning about direct oral anticoagulants for the first time.
(Btw, *no judgement here, we appreciate and welcome people of all seat preferences to read our post.)
Your professor eventually starts calling them DOACs (doh-acks), and you. are. hooked. Maybe it was only last semester, and you can still vividly remember bursting with excitement at learning about a new way to anticoagulate.
Or maybe it’s been years since you were in pharmacy school, and you’ve gotten to see first hand the clinical impact of DOACs where you practice. Or maybe, just maybe, you don’t even know what a DOAC is, and you’re thinking to yourself, “It’s only my third day out here, I don’t know.”
Well, not to worry, we’ve got you covered. The aim of this post is to review some basic “dos and don'ts” of DOACs in special populations. You see, as great as DOACs may be, they can’t be used in just anyone, and we hope this article helps to identify a few of those special populations, as well as go over possible alternative methods of anticoagulation when DOACs are not indicated.
We’re gonna cover a lot here, so if you’d like a downloadable and printer friendly version of this article, you can get that here. You’ll get a PDF that ALSO includes our Definitive Guide to Anticoagulants. It’s basically a comprehensive (but easy to read) resource that will make you an Anticoagulant Level: Expert in no time.
For the purposes of this post, the DOACs we’ll be focusing on are Xarelto (rivaroxaban), Eliquis (apixaban), Savaysa (edoxaban), and Pradaxa (dabigatran).
Briefly, rivaroxaban, apixaban, and edoxaban are factor Xa inhibitors, while dabigatran is a direct thrombin inhibitor. Additional details on them can be found in Anticoagulants: The Definitive Guide, so feel free to drop by if you need to brush up up the basics before going any further here.
Also, you should probably make your life a lot easier by checking out our Anticoagulant Cheat Sheet.
The populations we’ll be focusing on in this post are pregnancy, obesity, those with an artificial valve replacement, severe renal impairment, and hepatic impairment. However, please keep in mind there are other special populations (patients with a hypercoagulable state, altered gastrointestinal absorption, etc.) not mentioned here who still require special consideration when it comes to anticoagulation.
Without further ado, let’s get started!
Use of DOACs in Pregnancy
Do: low molecular weight heparins (LMWH) or unfractionated heparin (UFH)
Don’t: DOACs
Well, for being a post about DOACs, we certainly just poo-pooed the DOACs in the first special population, didn’t we… But alright, so how do we anticoagulate our pregnant patients?
First, it is important to note that pregnancy is a hypercoagulable state in and of itself. Pregnant patients have a 5 times increased risk of thrombotic events during gestation and for up to 12 weeks postpartum, thus requiring the need for proper assessment of anticoagulation. In data as recent as 2010, pulmonary embolisms were responsible for almost 10% of pregnancy-related deaths in the United States.
Typically, female patients of childbearing age are given strong precautions to not become pregnant while on warfarin due to its teratogenic effects (Category D). Monthly pregnancy tests and the use of contraception are highly recommended. During pregnancy, the safest option for the prevention and treatment of venous thromboembolism (VTE) is heparin; LMWH is preferred over UFH and is typically used throughout the majority of the pregnancy for women who meet criteria for either prophylactic or therapeutic anticoagulation.
The American Congress of Obstetricians and Gynecologists (ACOG) maintains a fantastic summary slide set of the decision-making process for determining which women meet criteria for anticoagulation versus those who do not. This bundle also has recommendations for dosing and management around procedures, such as epidural placement at the point of delivery.
A systematic review of LMWH proved it is both safe and effective in pregnancy (64 studies, 2777 pregnancies). According to the review, the rates of arterial and venous thrombosis were <1% and the rate of severe bleeding <2%; there were no maternal deaths or cases of heparin-induced thrombocytopenia.
Another study conducted by Desancho et al. measured outcomes in 89 pregnancies in 76 women who received LMWH during pregnancy from 2001 to 2010. The live birth rate was 97%, indicating successful pregnancy outcomes. The most common maternal complication was hypertension (28%), and the most common fetal complication was intrauterine growth restriction (11%). In the third trimester, patients were switched from LMWH to UFH to reduce the risk of spinal hematoma following regional anesthesia during delivery.
It should be no surprise, then, that based on current literature the 2012 CHEST guidelines recommend the following:
Substitute UFH or LMWH for warfarin during all trimesters of pregnancy
LMWH is recommended over UFH for prevention and treatment of VTE
The continuation of LMWH throughout pregnancy and for at least 6 weeks post partum is recommended in pregnant patients with an acute VTE (with special considerations surrounding the immediate delivery period)
Now that we’ve covered what we could use in pregnancy, let’s talk about why we can’t use DOACs.
For one, pregnant and breastfeeding patients were excluded from all major DOAC trials. As a result, the use of a DOAC in pregnancy is not recommended due to a lack of current research available from human clinical trials regarding efficacy and safety. Second, there is a possibility that the mechanism of action of the DOACs may not allow for sufficient anticoagulation during pregnancy.
Looking at all the clinical trials about DOACs in pregnancy like… (image)
Most DOACs are at least partially cleared by the kidneys, and renal elimination increases during pregnancy. It is possible that increased glomerular filtration rate during pregnancy results in subtherapeutic levels of the drugs remaining in the body. Furthermore, dabigatran works by reducing fibrinogen conversion to fibrin, and fibrinogen levels are elevated in pregnancy. At currently recommended doses, the amount of dabigatran a patient receives may not be sufficient in pregnant women.
You might be thinking to yourself, “Okay fine, but just because we don’t have human trial data doesn’t mean DOACs aren’t safe in pregnancy. Can’t we at least do animal studies to start?”
So glad you asked!
Most of the data we have available does come from animal studies. Overall, the results of these studies indicate DOACs cross the placenta and may be associated with numerous reproductive toxicities, including fetal growth restriction, gallbladder anomalies, post implantation loss, and overall reduced viability. Of the 233 unique reported cases of DOAC exposure in pregnancy, pregnancy outcomes for only 137 have been reported. Of the 137, thirty-one were miscarriages and seven showed abnormalities (embryopathy, dysmorphism, organ defects, etc.).
In terms of specific studies, here’s a summary of what we have so far:
Rivaroxaban: Reproductive studies performed in rats and rabbits showed placental changes, post-implantation loss, skeletal malformations, and cardiovascular defects at clinically relevant concentrations.
Apixaban: Apixaban is currently classified as category B in the United States, but its use should only be considered if the benefits outweigh the risks. The data collected demonstrated incidences of maternal bleeding, but none of these led to serious complications. There have been no reports of increased risk of fetal toxicity or malformation.
Dabigatran: At 5-10 fold higher plasma level concentrations compared to what’s usually given to patients, dabigatran was associated with decreased fetal body weight and embryofetal viability. Hard to interpret what would happen at normal doses, but are we going to start experimenting now…?
Edoxaban: In animal studies using significantly higher than maximum recommended human dose, edoxaban was shown to increase fetal variation and post-implantation pregnancy loss.
In summary, DOACs in pregnancy just plain don’t have enough data to be proven safe or efficacious, and the few studies we do have show an increased risk of fetal toxicity and reproductive side effects. LMWH and UFH are for now the safest anticoagulants to use in pregnancy.
Use of DOACs in Pediatric Patients
Do: dabigatran
Don’t: other DOACs
Dabigatran is the first FDA-approved DOAC for the prevention and treatment of venous thromboembolisms (VTE)s in pediatric patients. It is the first blood thinner that is available for pediatric patients to take orally. The indications include:
Treatment of blood clots in patients 8 years or older with VTE, who have taken an injectable blood thinner for at least five days
Prevention of recurrent blood clots in children who finished initial VTE treatment
Dabigatran comes in two formulations:
Pellets for children 3 months to 12 years of age
Capsules for 8 and older
Previously, dalteparin (Fragmin) was used to treat VTE in children and is available as a subcutaneous injection.
It is important to note that we start dabigatran after the patient has taken an injectable blood thinner for at least 5 days.
Most of our information for dabigatran comes from the DIVERSITY trial which enrolled 267 subjects up to 18 years of age. This was a randomized, open-label study that had three endpoints: no death due to blood clots, complete blood clot resolution, and additional blood clots. The study found that 45.8% (81) of patients in the dabigatran arm reached the composite endpoint.
Additional information came from a single-arm study which enrolled 214 children. These patients required additional anticoagulation after initial VTE treatment or finishing the DIVERSITY study. In this study, three (1.4%) patients experienced the recurrent clots.
Use of DOACs in Obesity
Do: warfarin/ apixaban/ rivaroxaban
Don’t: dabigatran
When treating obese patients (BMI > 30 kg/m2), warfarin is the safest option. While an increase in body mass index (BMI) has been associated with higher total weekly dose of warfarin, regular INR monitoring in this patient population ensures that patients have a sufficient amount of drug in their body to provide ample anticoagulation.
On the other hand, the level of anticoagulation provided by a DOAC cannot be as easily monitored. Furthermore, many of the landmark trials establishing the efficacy of DOACs excluded patients with a BMI of >40 kg/m2 or a weight of >120 kg. In terms of pharmacodynamics and pharmacokinetics, the concern is that increased body weight results in lower peak concentrations and shorter half lives, which could mean an overall insufficient amount of drug in the body.
Unfortunately, there are often cases where a patient’s profile and lifestyle are much better suited for a DOAC over warfarin. DOACs have simpler medication regimens, require fewer doctors’ visits, and are easier to manage in terms of diet and drug-drug interactions. So what if you explain to a patient that DOACs are not well-studied in obesity, but they insist, “That’s okay, it’s a risk I’m willing to take.”
In that case, there have been some promising trials comparing the efficacy of DOACs to warfarin in obese patients that may be worth looking into.
A retrospective, single-center cohort study published in August 2018 reviewed 64 adult patients with a BMI > 40 kg/m2 or weight > 120 kg who were on apixaban, dabigatran, or rivaroxaban for atrial fibrillation (AF) or flutter. The primary outcome was the incidence of ischemic stroke or transient ischemic attack (TIA). This study found that the annual incidence of stroke was 1.75% in the DOAC group compared to a rate of 2.07% in the warfarin group. The incidence of bleeding was also higher in the warfarin group (4.97%/year compared to 2.18%/year in the DOAC group). After analyzing the data collected for each DOAC specifically, the study concluded that apixaban or rivaroxaban might be possible alternatives to warfarin in obese patients, while dabigatran warrants more investigation.
A separate 2017 study looked retrospectively at apixaban vs. warfarin in 390 obese patients (average BMI 46.2 kg/m2) for safety and efficacy. The main indications for the medications were AF and VTE, and the measured clinical outcomes were VTE recurrence, stroke, and bleeding. Although this study was not powered to show statistical significance, it did demonstrate similar efficacy between warfarin and apixaban.
The rate of VTE recurrence was similar in both groups (1.7% in the apixaban group vs. 1.1% in the warfarin group, p=0.76). For prevention of stroke in patients with AF, there was no statistically significant difference between groups (0.8% in the apixaban group vs. 2.4% in the warfarin group, p=0.31) - although the apparent difference in those numbers is rather interesting. In addition, apixaban was associated with a lower bleeding risk (0.6% in the apixaban group vs. 4.3% in the warfarin group, p=0.02). The overall low recurrence rate of VTE and stroke incidence in the apixaban group is promising for its place in therapy in the obese population.
So, if you’ve weighed the risks vs. benefits with the patient and decide to use a DOAC, it’s not an unreasonable choice. However, no need to go making up doses, just use the medication at labeled doses.
Also, understand that there is potentially a higher chance of the patient failing DOAC therapy. But if you’re gonna go for the DOACs, the following monitoring parameters may be useful:
Check anti-FXa levels for apixaban, edoxaban, and rivaroxaban
Check ecarin time or dilute thrombin time with appropriate calibrators for dabigatran
Check mass spectrometry drug level for any of the DOACs
If levels are normal, DOAC therapy can be continued. In contrast, if levels return below therapeutic range, switching to warfarin is recommended rather then attempting to dose adjust the DOAC.
In summary, DOAC use seems to be on the rise in obese patients despite a lack of clinical trial data. While warfarin would be the preferred agent, there is an increasing amount of data indicating that DOACs might be reasonable alternatives, especially in patients who find it hard to keep up with the strenuous monitoring that warfarin requires or whose lifestyle makes it impossible to visit a provider regularly and manage complex medication regimens.
Use of DOACs in Patients with Artificial Heart Valves
Mechanical Heart Valves
Do: Warfarin + low dose aspirin
Don’t: DOAC
Bioprosthetic Heart Valves
Do: Warfarin + low dose aspirin OR possible off-label apixaban or edoxaban
Don’t: Rivaroxaban or dabigatran
There are two types of artificial valves that call for different anticoagulation therapies: mechanical and bioprosthetic valves. So, in keeping with theme of this post, do we or don’t we DOAC? Spoiler alert: you might want to tell your patients with mechanical valves to start watching their morning spinach smoothie intake. That’s right…
For all you 80s babies growing up in the 90s…you know you love this, just admit it.
Anticoagulation in Patients with Mechanical Heart Valve Replacements
Image this, it’s 2009, and the RE-LY study for dabigatran in AF has just been published. People are going absolutely gaga over the first new way to anticoagulate orally since ancient warfarin.
And then, this…
The RE-ALIGN trial investigating dabigatran in mechanical heart valves was published in 2013. It showed an increased rate of thromboembolic and bleeding events with dabigatran in patients with mechanical valves when compared to warfarin. These increased rates prompted the study to be prematurely discontinued. #fail
In patients randomized to dabigatran, increased occurrences of stroke (5%), myocardial infarctions (2%), and valve thrombosis (3%) were observed. No cases of any of these events were reported in patients randomized to warfarin. The majority of these adverse events were seen in patients who initiated dabigatran 7 days after valve implantation. Events were less common when dabigatran was initiated more than 3 months after valve implantation. While we believe the timing of initiation may be a consideration factor, dabigatran, nonetheless, is not indicated in patients with mechanical valve replacements.
While we are currently ruling out dabigatran as an option for these patients, we have yet to see any additional trials evaluating the other DOACs on the market. This makes life-long therapy of warfarin with aspirin 75 to 100 mg daily our treatment of choice.
Now that we’ve chosen our drug of choice, how do we pick an INR goal?
First, we need to ask a few questions:
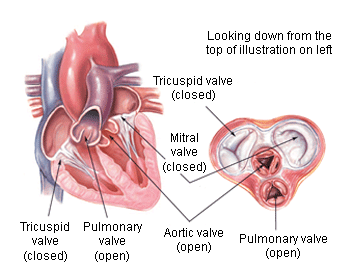
Which valve is being replaced? Aortic or mitral?
A quick review of heart valves and locations. Note that in this post, we’re talking about the mitral (or bicuspid) and aortic valves, both on the left side of the heart. (Image)
2. What type of prothesis is being used? Mechanical or bioprosthetic?
Yes, we’re pharmacists, but it does help to know what some of these things look like! I mean, that caged-ball contraption positively screams blood clot doesn’t it??? (Image)
3. Does the patient have any other risk factors for thromboembolism? AF, previous thromboembolism, left ventricle dysfunction, and/or hypercoagulable condition?
After assessing these pieces, we can start to form our mechanical valve INR goal recommendations according to the following guidelines:
Goal INR 2-3 (target 2.5)
Patients with mechanical bileaflet aortic valve replacement (AVR) with no risk factors
Patients with current-generation single-tilting disc AVR with no risk factors
Goal INR 2.5-3.5 (target 3.0)
Patients with mechanical AVR with risk factors
Patients with older-generation mechanical AVR (such as ball-in-cage)
Patients with mechanical mitral valve replacement
Anticoagulation in Patients with Bioprosthetic Heart Valve Replacements
There is limited data available when it comes to the use of DOACs in patients with bioprosthetic valves. Here is a summary of the trials we have so far:
Rivaroxaban: Not recommended. The GALILEO trial resulted in increased thrombotic and bleeding events with the use of rivaroxaban in TAVR (transcatheter aortic valve replacement) patients. The trial was prematurely discontinued in August 2018.
Apixaban: Possible off-label use. The ARISTOTLE trial performed a subanalysis of the 260 patients with a history of valve surgery included in the landmark study. Of these 260 patients, 82 had bioprosthetic valves (41 patients in the apixaban group and 41 patients in the warfarin group). Two instances of stroke were reported in the apixaban group (none in the warfarin group). Overall, however, there were no statistically significant differences for major bleeding, stroke or systemic embolism, all-cause death, or cardiovascular death. Still pretty small numbers to base any patient decisions on though…more info needed!
Edoxaban: Possible off-label use. The ENGAGE AF trial conducted a pre-specified subgroup analysis of patients with bioprosthetic aortic and mitral valves (191 patients out of about 20,000) in their comparison of edoxaban and warfarin. Edoxaban patients had similar outcomes to warfarin in terms of stroke, systemic embolism, and bleeding. But again, small numbers…need more info!!
Dabigatran: Not recommended. Has not been evaluated.
Real footage of us keeping up with the ENAVLE trial (Image)
P.S. If you want to know more about DOACs in patients with a bioprosthetic valve replacement, the ongoing ENAVLE trial might be worth looking into!
Overall when anticoagulation is required for bioprosthetic valve replacements, warfarin with a goal INR of 2-3 is our treatment of choice.
(FYI, an exception is made for patients with mechanical On-X AVR and no thromboembolic risk factors. These patients may have a lower target INR of 1.5 - 2, but we still warfarin.)
Aspirin 75 - 100 mg daily may be added to either population.
Of note, bioprosthetic valve replacements have a lower risk of thrombosis than mechanical valves and are not indicated for lifelong anticoagulation. Rates of thrombosis are highest in the first 3 months after a valve replacement, therefore duration of therapy is typically 3-6 months.
Use of DOACs in Patients with Chronic Kidney Disease on Dialysis
Do: Warfarin or apixaban (CrCl dependent)
Don’t: other DOACs (CrCl dependent)
Me: I just hope this CrCl stops dropping.
CrCl:
Due to its hepatic metabolism, warfarin has been widely chosen as the method of anticoagulation for patients with renal impairment. DOACs, on the other hand, have specific recommended CrCl cut offs for use or adjustment, but they depend on indication. For those dose adjustments, feel free to refer to Anticoagulants: The Definitive Guide. It probably also wouldn’t hurt to review where CrCl comes from in this Kidney Beans post since this is integral for dosing…
When a preceptor comes over to verify my renal dose adjustments. (Image)
Renal adjustments for most DOACs begin at a CrCl of 30-50 mL/min, and their use is not recommended in severe renal impairment or dialysis. The exception to this rule is apixaban, which currently has dosing recommendations for severe renal impairment and/or dialysis (although this population was excluded from the landmark ARISTOTLE trial).
So how did that recommendation happen? Don’t worry, we’ll get there.
While warfarin is old as dirt and is commonly used in renal impairment, its use is actually still controversial when it comes to the data, especially for dialysis patients. Risk and benefit must be weighed!
While some trials have showed benefits of oral anticoagulants in dialysis patients, others have shown it to increase the risk of strokes.
For example, the SPAF 3 trial (Stroke Prevention of Atrial Fibrillation) studied over 500 dialysis patients for 2 years and concluded that the full dose warfarin group had a 76% reduced risk of ischemic strokes or systemic embolisms compared to control. In contrast, a retrospective analysis of 1671 hemodialysis patients on warfarin vs. dual antiplatelet therapy vs. no therapy found that the risk of a stroke was increased 2 fold in patients on warfarin as compared to the other two groups. The highest risk of stroke occurred in patients with the most elevated INR values and those whose INR values were not regularly monitored.
Confusing, right? And yet there we are, recommending warfarin for our dialysis patients… Because what else can we do when they have AF or a blood clot?!? We can’t keep them in the hospital forever on a heparin infusion.
Sigh.
In terms of recent DOAC trials in this population, apixaban seems to be the most promising and well studied.
In a 2017 retrospective, matched-cohort study of 357 patients with CrCl < 25 mL/min or SCr > 2.5 mg/dL or receiving peritoneal/hemodialysis, apixaban was compared with warfarin for safety, with the primary outcome being major bleeding and secondary outcomes being the occurrence of ischemic stroke and recurrent VTE. There was no statistical significance in the occurrence of major bleeding events between the apixaban and warfarin groups (9.6% and 17.8%, respectively, with p=0.149). Furthermore, in the apixaban arm, dialysis patients had similar bleeding rates compared with non-dialysis patients. Regarding occurence of stroke or VTE, no statistical significance was found between the warfarin and apixaban groups. Although the study was limited by its sample size and short duration, it showed the potential apixaban has in being an alternative to warfarin in patients with severe renal impairment.
In another 2018 retrospective study of 124 hemodialysis patients, there were significantly fewer overall bleeding events in the apixaban group compared with warfarin (18.9% vs. 42%, p=0.01). Major bleeding was also significantly less in the apixaban group (5.4% vs. 22%, p=0.01). There was no difference in recurrent strokes between groups (none in either cohort), and apixaban may have had fewer recurrent VTEs (4.4% vs. 28.6%, p=0.99).
Finally, in this large 2018 analysis of apixaban in dialysis-dependent end-stage renal disease patients with AF, 2351 apixaban patients were retrospectively compared with over 23,000 patients on warfarin. It should be noted that only a small number of patients (~5-6%) in this study were on peritoneal dialysis. Most were hemodialysis-dependent. No difference was found in efficacy (aka stroke or systemic embolism), but patients on apixaban had significantly less major bleeding. This study also indicated that standard dosing of apixaban 5mg BID is warranted rather than empirically reducing to 2.5mg BID for dialysis.
This conclusion is in contrast with an analysis of the pharmacokinetics and pharmacodynamics of apixaban in hemodialysis patients. A 2017 study published in the Journal of the American Society of Nephrology looked at seven patients on hemodialysis taking apixaban 2.5 mg or apixaban 5 mg. Even with the lower dose, significant accumulation was observed within 8 days, and the area under the curve increased 2-5 fold (p<0.001). On the 9th day, patients underwent hemodialysis, where apixaban levels were monitored hourly in 6 of the patients. The study concluded that only 4% of apixaban was removed during dialysis, meaning that the procedure has minimal effects on drug concentrations in the body. The study also found that the twice daily 2.5 mg apixaban dose in hemodialysis patients resulted in similar drug levels as patients who qualified for a dose reduction in previous trials. In terms of clinical use, the findings indicate that the lower dose of apixaban is more favorable than the 5 mg twice daily dose, which produced supratherapeutic levels (>90th percentile of predicted levels).
Based on these studies, if warfarin is not an option in severe renal impairment, apixaban is the next and only DOAC of choice at this time. Close attention should be paid to the type of dialysis (hemo- vs. peritoneal) and stability of dialysis regimen.
IMPORTANT: These conclusions do not necessarily apply to patients with severe renal impairment due to acute kidney injury (AKI). AKI is a period of fluctuating renal function, and none of the above studies has investigated DOAC use during this time.
Use of DOACs in Patients with Hepatic Impairment
Do: Warfarin or DOACs (with limitations)
Don’t: DOAC in moderate to severe hepatic impairment
Hepatic impairment comes with increased risk of both thrombosis and bleeding. How is that even possible? Well, we’re glad you asked.
The liver plays an elaborate role in regulating various coagulation factors, and when the liver can’t function properly, the coagulation pathway goes haywire. This fancy chart sorts out which factors lead to either increased thrombosis or bleeding in the setting of hepatic impairment.
Warfarin has commonly been used in thrombotic treatment and prevention in patients with hepatic impairment. While it may be common, however, there are several complications to note with its use in this population.
First, there’s the fact that warfarin’s main metabolic pathway is via CYP450 pathways in the liver. Decreased liver metabolic functions may mean highly variable INRs depending on how much warfarin is getting eliminated (or not).
Next, let’s add the fact that warfarin’s MOA is to impact activation of factors VII, IX, X, and II. So if a patient has baseline liver dysfunction and isn’t synthesizing these clotting factors as well, how does that impact warfarin’s action? And how does this balance with the increased prothrombotic factors from the chart above?
Additionally, a well-defined INR goal for patients with hepatic impairment is lacking. Many patients with liver dysfunction already have an elevated baseline INR from the above considerations. So while there is a set goal for the general population with AF and VTE (goal INR 2-3), this can’t really be adopted for those with hepatic impairment. If a patient’s baseline INR is 1.7, does raising that to 2 with warfarin for AF have the same impact as someone who started at a normal INR of 1? And what are the implications for bleeding?
SO UNCLEAR!
Studies reviewing the efficacy and safety of DOACs in hepatic impairment include pharmacokinetic studies, case reports, and small observational studies. Thus, there is minimal data from clinical trials. But instead of dwelling on what we don’t have, we can discuss what we do have.
A retrospective 2019 clinical study examined apixaban, dabigatran, rivaroxaban, and warfarin in cirrhotic patients with AF. Of note, in each of the DOAC groups, the more conservative dosing strategy was used in the majority of patients (e.g., apixaban 2.5mg instead of 5mg). The DOACs vs. warfarin groups received comparable rates of ischemic stroke or systemic embolism events (3.2% vs. 3.7% per year) and intracranial hemorrhage events (1.0% vs. 1.6% per year). In contrast, the DOAC group had a lower risk of gastrointestinal bleeding (1.9% vs. 3.6% per year) and all major bleeding (2.9% vs 5.4% per year). Interesting, and thank goodness we’re starting to get some organized data about this, but still so many questions…
Rates of hepatic metabolism vary between DOACs and may affect efficacy and safety. From greatest rate of hepatic metabolism to least: apixaban (75%), rivaroxaban (65%), edoxaban (50%), and dabigatran (20%). Specific recommendations for each DOAC are as follows:
Big Takeaways
Well guess what, you made it to the end. (Or if you were actually just scrolling to the end to see how long this post actually is - we guess there’s that too. But you missed some GREAT stuff!!) Either way, here are some major takeaways:
In pregnancy, limited data is available, but for now avoid DOACs. LMWH is best, especially earlier in pregnancy.
Consider the risk of lower DOAC concentrations, and therefore efficacy, in patients with a BMI > 40 kg/m2.
If you are asked to anticoagulate for a mechanical valve, go straight to warfarin.
DOAC data in bioprosthetic valves is limited, but use may be considered.
Lastly, keep in mind the various dose adjustments for renal or hepatic impairment based on each DOAC. Apixaban may be an option in hemodialysis.
Happy anticoagulating! :)
Want a downloadable and printer friendly version of this article? Well good news, you can get that here. You’ll get a PDF that ALSO includes our Definitive Guide to Anticoagulants. It’s a comprehensive (but easy to read) resource that will make you an Anticoagulant Level: Expert in no time.