The Pharmacist's Guide to Oral Diabetes Drugs
*This article is currently under construction. Please see the ADA’s Standards of Care in Diabetes 2025 for most current recommendations.*
Courtney’s Note: It’s time for another long-overdue article update! Akin to previous updates, I’m going to try to retain as much of the original author’s content and voice as possible. These articles are a lot of work, and we appreciate the time and energy put into them. Today, we’ll be working with the brand-spanking-new guidelines put out by the American Diabetes Association (ADA) to learn more about oral drugs used to treat diabetes. We stan an organization that updates their guidelines annually!
Brandon’s Note: Cara Clayton is a recent graduate from the University of Texas at Austin College of Pharmacy. You may remember her as the person who wrote that excellent overview of sickle cell disease for us.
Now that she's graduated, Cara's back at it again with this fantastic overview of oral diabetes drugs (seriously, this post is really good).
Cara is about to start her PGY1 residency at Scott & White Cancer Center & Medical Center in Temple, Texas. Pending successful completion of her PGY1 residency, she hopes to pursue a PGY2 residency in Oncology and become a Board Certified Oncology Pharmacist.
I hope that you all get as much out of reading this post as I did. Thanks Cara!
From the 1950s to the 1990s, there were only two classes of oral medications used to lower blood sugar: sulfonylureas and biguanides. Think about that. There was almost no development in this area for four decades!
But as the prevalence of type 2 diabetes mellitus (T2DM) began increasing, there was a corresponding increase in the types of oral glucose-lowering medications available.
It's really a classic supply/demand curve.
Beginning in the late 1990s and early 2000s, new oral drugs for the treatment of T2DM began to arrive on the scene.
Today, available classes of oral glucose-lowering agents include:
Sulfonylureas (second generation)
Biguanides
Meglitinides
Thiazolidinediones
DPP-4 inhibitors
SGLT2 inhibitors
GLP-1 RAs
Alpha-glucosidase inhibitors
Bile acid sequestrants
Dopamine-2 agonist (bromocriptine)
And in some of these classes, there are multiple medications. Meaning T2DM can be an overwhelming subject to learn for a pharmacist. In this post, I hope to make it easier for you.
However, before diving further into the treatment of diabetes mellitus (DM), let’s briefly discuss its background, pathophysiology, diagnosis, and evaluation.
Diabetes Mellitus Background and Pathophysiology
DM consists of a group of metabolic disorders that result in hyperglycemia (or high blood glucose). This hyperglycemia is often caused by defects in insulin secretion or action.
You may be thinking, “High blood sugar is no big deal, right?”
WRONG!
According to the 2024 National Diabetes Statistics Report:
Diabetes was the eighth leading cause of death in the U.S. in 2021.
Total direct estimated costs of diagnosed diabetes increased from $227 billion in 2012 to $307 billion in 2022. Total indirect costs increased from $89 billion to $106 billion in the same period.
About 8.9% of the US population (29.7 million people of all ages) had diabetes in 2021.
About 97.6 million adults aged 18 years or older had prediabetes in 2021.
There are various types of DM, the most common of which include type 1, type 2, and gestational diabetes. Additionally, there are other lesser-known types of DM, including:
Drug-induced DM (think steroids, protease inhibitors, and calcineurin inhibitors such as tacrolimus)
DM caused by genetic defects of beta cell function (pancreatic cells responsible for insulin production and secretion)
Diseases of the exocrine pancreas (think cystic fibrosis and pancreatitis)
Although this article will focus mainly on T2DM, here's the gist of what you need to know about the three most common types of DM:
Type 1 DM (T1DM): An autoimmune disease that causes the beta cells of the pancreas to be destroyed. This in turn prevents the body from producing enough, or any, insulin. As time goes on and more insulin-producing cells in the pancreas are killed off, the body loses its ability to control blood glucose levels and symptoms of diabetes begin to appear.
T1DM is often diagnosed in childhood.
T1DM is sometimes referred to as “insulin-dependent diabetes”.
Type 2 DM (T2DM): A metabolic disorder that results when the body is ineffective at using the insulin it has produced, or is unable to produce enough insulin
T2DM is often diagnosed in adulthood. However, it is now becoming more common in children and teenagers.
T2DM accounts for 90-95% of all DM cases.
Gestational DM (GDM): Diabetes that is diagnosed in the second or third trimester of pregnancy in a mother who did not have preexisting type 1 or type 2 DM.
Patients that develop GDM have an increased risk of developing T2DM later on.
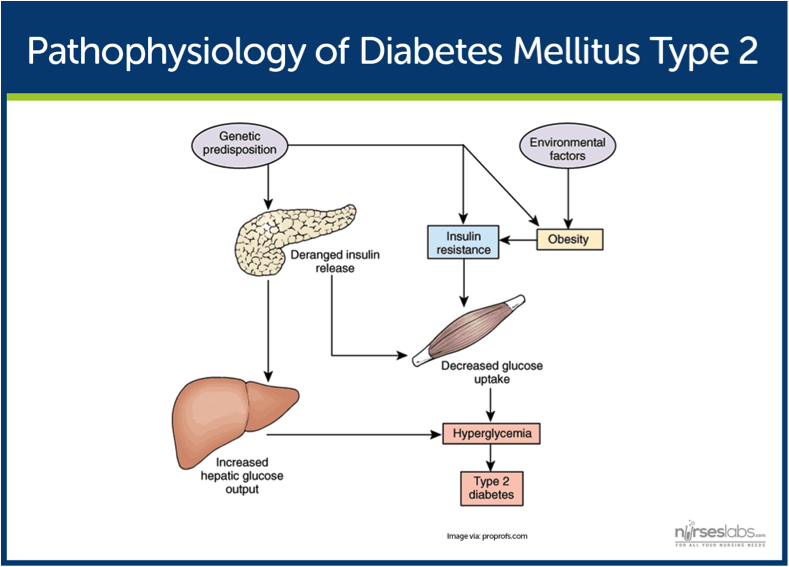
Alright, let's move on to the pathophysiology of type 2 diabetes.
The primary problem in patients with T2DM is insulin resistance. This resistance is usually caused by genetic predisposition and certain risk factors.
What risk factors? I'm glad you asked.
Risk factors for T2DM in adults include:
Overweight (BMI > 25 kg/m2 )
First degree relative with T2DM
Hypertension
Cardiovascular disease
Physically inactive
History of gestational diabetes
Hyperlipidemia
Belonging to a high-risk race/ethnicity (African American, Latino, Native American, Asian American, or Pacific Islander)
And, while we're at it, risk factors for T2DM in children and adolescents include:
Overweight or obese
Maternal history of DM or GDM during the child’s gestation
T2DM in a first or second degree relative
Belonging to a high-risk race/ethnicity (African American, Latino, Native American, Asian American, or Pacific Islander)
Acanthosis nigricans (this is a brown to black velvety hyperpigmentation of the skin which often suggests insulin resistance)
Hypertension
Dyslipidemia
Polycystic ovarian syndrome (PCOS)
Small birth weight for gestational age
So, in an actual patient, the combination of genetic predisposition and the above risk factors leads to less insulin secreted by the pancreas, more glucose pumped out by the liver, and less glucose uptake by your peripheral muscle and fat cells. The end result here is more glucose in your blood stream (hyperglycemia), and T2DM.
So, why is DM such a big deal? In addition to being the eighth leading cause of death and costing the U.S. healthcare system a lot of money, it can also cause a lot of long-term complications.
The risk of complications rises in a linear fashion with the length of time someone has had DM, as well as the lack of blood sugar control. More simply put:
↑ time with DM diagnosis = ↑ risk of complications
↓ control of blood sugar = ↑ risk of complications
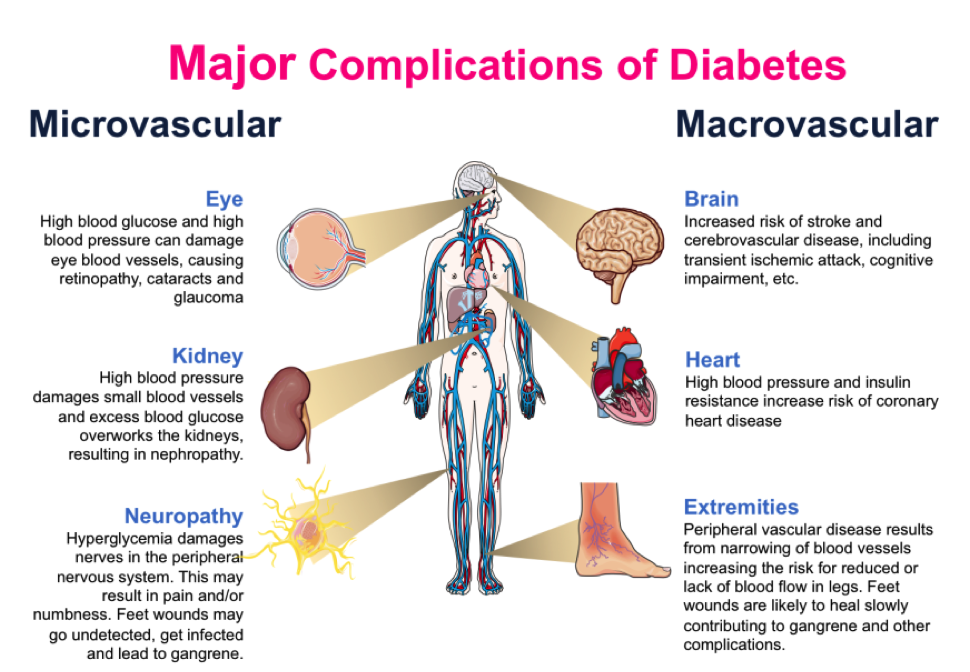
Complications of DM can be broken down into two main categories: microvascular (involving small vessels, such as capillaries) and macrovascular (involving large vessels, such as arteries and veins).
Check out this image for examples of both microvascular and macrovascular complications:
The Diabetes Control and Complications Trial is a prospective, randomized-controlled trial that showed that better blood sugar control reduces microvascular complications of diabetes.
Additionally, the U.K. Prospective Diabetes Study found that after 10 years of follow up, patients managed with "intensive glycemic control" had significant decreases in myocardial infarction and all-cause mortality.
Let's switch gears a little bit, and move on to the diagnosis and evaluation of DM. After that, we can finally move on to the fun part: the medications.
Diagnosis and Evaluation of Diabetes Mellitus
Symptoms of DM will vary based on the type of DM and how high the blood sugar is.
The "classic" symptoms of DM are:
Polyuria (frequent urination)
Polydipisa (increased thirst)
Polyphagia (increased hunger)
Other symptoms include fatigue, weight loss, blurry vision, slow wound healing, and frequent infections.
Symptoms can appear suddenly or develop slowly over time depending on the type of DM. In T1DM, symptoms usually appear suddenly, with patients often being diagnosed after a trip to the emergency department with diabetic ketoacidosis (DKA) or hyperosmolar hyperglycemic state (HHS). In contrast, T2DM tends to develop over months or years.
The ADA recommends consideration of testing for T2DM in adults of any age with BMI ≥ 25 kg/m2 (or ≥ 23 kg/m2 in individuals of Asian ancestry), and at least 1 additional risk factor (see the risk factors I listed above). Testing for all other adults should begin at 35 years old.
Patients with prediabetes and those diagnosed with GDM should be tested on a regular basis (yearly and every 1-3 years, respectively). Those taking high-risk medications (e.g. second generation antipsychotics (SGAs)) and those that have high-risk comorbidities (e.g. HIV) should be monitored closely for the development of T2DM.
Screening should be considered for children and adolescents who are overweight or obese (BMI > 85th percentile for age and sex) and have at least 1 additional risk factor (again, see the risk factors I listed above).
Tests for DM include:
Hemoglobin A1c test - Measures the percent of blood sugar attached to hemoglobin and indicates your average blood sugar level for the past three months. Because you're measuring glycated hemoglobin, conditions that affect red blood cell turnover (hemolytic anemia, pregnancy, end-stage renal disease, recent blood transfusion) may result in inaccurate A1c results.
Random blood sugar test - A random blood sample taken regardless of when you last ate.
Fasting plasma glucose (FPG) - A blood sample taken after fasting for at least 8 hours.
Oral glucose tolerance test (OGTT) - Almost like a combo of the fasting and the random blood sugar tests. You fast overnight and measure your fasting blood sugar level for a baseline. Then, you drink a glucose drink and measure your blood sugar levels occasionally during the next two hours (i.e. a 2-hour plasma glucose (2-h PG)).
Yummy Sugar Drinks (Image)
The results of these tests tell you if your blood sugar is normal (normoglycemia), or if you're prediabetic or diabetic.
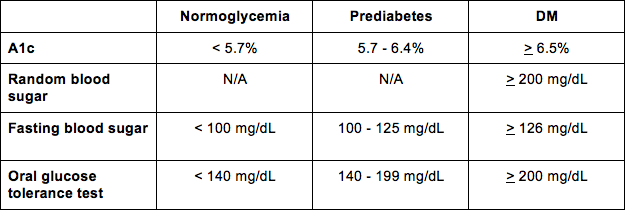
And for your reference, here are the diagnostic criteria so you can interpret the results of each test for your patient.
An important point is that diagnosis of DM in asymptomatic patients requires two separate readings (on separate days) higher than the cutoff value. If possible, it is recommended to repeat the same test when obtaining these two separate readings. So you should try to use two A1c tests, or two fasting blood sugar tests, etc.
If the results are normal, patients can wait up to three years before testing again. If results are borderline, testing will need to be done more frequently.
If the patient is experiencing classic symptoms of hyperglycemia or a hyperglycemia crisis, one random glucose ≥ 200 mg/dL is sufficient to diagnose DM.
After initial diagnosis and evaluation of T2DM, follow-up visits should occur every 3-6 months, and then annually. Additionally, A1c should be measured every 3 months until it reaches the patient’s individual goal, then it can be measured every 6 months.
So now that we know where the cutoffs for diagnosis are, what about treatment? What goals are we hoping to achieve with medication therapy?
Glycemic goals for nonpregnant patients with T2DM include:
A1c: < 7%*
Fasting blood sugar: 80 - 130 mg/dL
Peak postprandial blood sugar: < 180 mg/dL
*A1c goals may vary depending on the patient. Newly diagnosed patients who have no significant heart disease, a longer life expectancy, or who are managed only with lifestyle changes or metformin may have a stricter A1c goal of < 6.5%.
Conversely, patients who are older, have a long history of diabetes, extensive comorbidities, or a history of severe hypoglycemia may have a less strict A1c goal of < 8%.
For all you visual learners out there, here’s an image that sums up the criteria for A1c goals:
Alright, it's finally time to move on to treatment of DM. Again as a quick note, this article will only focus on oral DM therapies. I'll leave injectable treatments for another day.
Editor's Note: We've made a Diabetes Cheat Sheet that handily summarizes everything in this post and more. Check it out by clicking the link below!
Oral Treatments for Diabetes Mellitus
Before getting straight into the drugs, we should review the other non-pharmacologic treatments for DM. Diet and exercise play a huge (and essential) role in the management of DM, so I'd be remiss to leave them out.
Lifestyle modifications include nutrition therapy, physical activity, smoking cessation, and weight loss (if needed).
Nutrition therapy - All patients with DM need an individualized eating plan and medical nutrition therapy, preferably provided by a registered dietician. Nutrition therapy is important to improve basically all elements of DM (overall health, body weight, blood sugar, blood pressure, lipids), and to prevent complications of diabetes.
Physical activity - A goal of at least 150 minutes of "moderate to vigorous" aerobic exercise per week, spread out over at least 3 days. Patient's should avoid going more than 2 consecutive days without physical activity. Physical activity helps with glucose control, weight loss, and reducing the risk of cardiovascular disease.
Smoking cessation - Smokers (and people regularly exposed to secondhand smoke) have an increased risk of cardiovascular disease, microvascular complications, and premature death. So, it goes without saying that cessation counseling (and pharmacologic therapy if necessary) are part of the treatment plan for DM.
Weight loss - The Look AHEAD trial showed that loss of 5-10% of body weight helps to improve fitness, CVD risk, A1c, and decrease the use of antihypertensives, cholesterol-lowering medications, and glucose-lowering agents. Weight loss can also reduce the severity of depression and obstructive sleep apnea. However, long-term weight loss is difficult for many DM patients since many glucose-lowering agents have the nasty side effect of weight gain. Nutrition therapy and physical activity can help here.
Now, moving on to pharmacologic treatment. As I mentioned earlier, there are many different oral glucose-lowering agents. These agents can be used alone or in combination, and are often recommended at various stages of diabetes.
Additionally, medications in the same class often have different side effects, monitoring parameters, and counseling points (which makes studying these drugs suuuuuper fun).
I'll do my best to break each class down to the essential clinical pearls and keep it to the tl;dr info, for your learning enjoyment.
As a reminder, these are our oral diabetes drug classes:
Sulfonylureas (second generation)
Biguanides
Meglitinides
Thiazolidinediones
DPP-4 inhibitors
SGLT2 inhibitors
GLP-1 RAs
Alpha-glucosidase inhibitors
Bile acid sequestrants
Dopamine-2 agonist (bromocriptine)
Sulfonylureas
Sulfonylureas are broken down into first and second-generation agents. First-generation sulfonylureas aren't used much anymore because they are less potent and have a worse safety profile (i.e. more adverse effects) compared to the second generation agents.
Your First-generation sulfonylureas are chlorpropamide, tolbutamide, and tolazamide.
Second-generation sulfonylureas include glyburide, glipizide, and glimepiride.
Because the first-generation agents aren’t commonly used, I'm going to focus on the second-generation agents.
Sulfonylureas exhibit their glucose-lowering effects by binding to the sulfonylurea receptor 1 (SUR 1) on the surface of pancreatic beta-cells, which leads to insulin secretion.
Their pharmacologic effect is correlated with how well the beta cells are functioning. Since beta cells’ functionality decreases with progression of T2DM, the effectiveness of sulfonylureas will decrease over time. At this point, you can either add a second drug or switch to a different drug class.
Sulfonylureas can lower the A1c about 1% - 2%, which (as you'll see soon) is pretty impressive for an oral agent. However, they often cause hypoglycemia and weight gain. They also tend to accumulate in renal failure, which makes their side effect profile (especially hypoglycemia) even worse.
Because of this their tendency to cause drops in blood sugar, a big counseling point for patients is to always eat with their sulfonylurea (and to skip their dose if they skip a meal).
It’s also worth noting that sulfonylureas contain a sulfa moiety. Because of this, there is a theoretical concern for cross-reactivity in patients with a sulfa allergy (although the incidence is low and most patients tolerate them just fine!).
Glimepiride [Amaryl]
Glimepiride comes in a tablet form that may be dosed up to 8 mg PO per day. Some evidence suggests that glimepiride may be the preferred sulfonylurea in patients with coronary heart disease, while other evidence shows no difference between agents in CHD.
Glipizide [Glucotrol]
Glipizide comes in both immediate-release and extended-release tablets. Immediate-release glipizide technically can be dosed up to 40 mg PO per day, but we usually try to keep the dose of glipizide (both IR and ER) below 20 mg PO daily due to higher risk of side effects at higher dosages.
Glipizide is the preferred sulfonylurea in renal insufficiency. It is also preferred in elderly patients because it is metabolized to inactive metabolites, which may decrease its risk of hypoglycemia.
Glyburide [Diabeta], [Micronase], [Glynase PresTab]
Glyburide comes in a conventional tablet form [Diabeta], [Micronase] and a micronized tablet form [Glynase PresTab]. Conventional glyburide may be dosed up to 20 mg PO per day, while micronized glyburide maxes out at 12 mg per day. It’s important to note the two forms are not bioequivalent.
Sulfonylureas are generally not recommended in pregnancy because they are known to cross the placenta and can cause neonatal hypoglycemia. Our go to drug for GDM is insulin, but sulfonylureas may be an appropriate alternative when insulin is not an option. In these cases, we usually reach for glyburide since it has the most data in pregnancy.
Biguanides (AKA Metformin [Glucophage])
The only available biguanide is metformin (phenformin, we hardly knew ye). It works by decreasing hepatic glucose production and by increasing peripheral glucose uptake and utilization. As an added bonus, metformin has also been found to decrease risk of cardiovascular events and death.
Metformin used to be the golden child of T2DM treatment. In fact, prior to the 2022 revision of the ADA diabetes guidelines, it was recommended as initial therapy for all newly diagnosed patients (barring any contraindications, of course). These days, first-line therapies are guided by patient-specific factors like comorbidities and treatment goals. So, while it’s still very much a viable first-line option, it’s no longer the star of the show. Sad day.
So who shouldn’t use metformin? Glad you asked.
Although not as toxic as its extinct cousin phenformin, metformin has a risk of causing or contributing to lactic acidosis. It is contraindicated at an eGFR < 30 mL/min/1.73m2, and in patients with acute or chronic metabolic acidosis (including DKA).
The renal restrictions used to be a little more strict for metformin, but they've relaxed recently. Previously, there were hard serum creatinine (SCr) cutoffs for use (1.5 mg/dL for men, and 1.4 mg/dL for females). However, recent-ish data has shown that metformin is reasonably safe at those creatinine levels, and so we now use the eGFR of 30 mL/min/1.73m2 as a cutoff. Renal function should be monitored when eGFR is between 30 and 45 mL/min/1.73m2, and dosage may need to be adjusted.
As a final note, metformin needs to be held for 48 hours before and after IV contrast, as IV contrast is extremely nephrotoxic and an acute renal failure may lead to acidosis from metformin. I'll talk more about the lactic acidosis bit in a minute.
Metformin lowers A1c by 1.5%-1.7%. It is supplied in many combination products with other oral DM drugs (see the tl;dr pharmacy Diabetes Cheat Sheet for help memorizing these), and as an immediate-release tablet and an extended-release tablet.
Metformin may technically be dosed up to 2,550 mg/day, but we usually max it out at 2,000 mg PO per day due to some pretty nasty adverse GI effects (diarrhea, nausea, vomiting, and flatulence) at higher dosages. In fact, we usually start with a lower dose (500 mg PO BID) and titrate our way up to mitigate the GI issues. Taking metformin with food will also help.
Don’t want lactic acidosis? Just be anemic. Problem solved! Image
Alright, let’s circle back to lactic acidosis. Metformin has a black-box warning for it.
Risk factors include renal or hepatic impairment, excessive alcohol intake, age > 65 years, and concomitant use with carbonic anhydrase inhibitors or iodinated contrast.
Symptoms of metformin-associated lactic acidosis are often subtle and nonspecific, so it's hard to recognize if you're not looking for it. These symptoms include muscle aches, respiratory distress, drowsiness or altered mental status, and abdominal pain.
If acidosis is suspected, metformin needs to be discontinued. It can land patients in the ICU depending on the severity.
Metformin may also cause vitamin B12 deficiency when used long-term. And, unfortunately, one of the hallmark symptoms of DM (peripheral neuropathy) is the main symptom of B12 deficiency. So we tend to monitor B12 serum periodically in patients with peripheral neuropathy or anemia.
Another important clinical pearl is that metformin may restore spontaneous ovulation. Because of this, it's actually used as a treatment for polycystic ovary syndrome (PCOS). If your patient is of childbearing age and they aren’t planning on having children any time soon, you may want to remind them to stay on top of their contraception!
Meglitinides
The meglitinides consist of repaglinide and nateglinide. They increase insulin release from the pancreas in a glucose-dependent manner. Since glucose is required for insulin to be released, meglitinides should be taken 15 - 30 minutes before meals.
You can think of them kind of like fast acting sulfonylureas (but with a lower risk of hypoglycemia since meglitinides are glucose-dependent).
Meglitinides typically lower A1c about 1.7%. Adverse effects include weight gain, headache, hypoglycemia, and upper respiratory infections. Because of the risk of hypoglycemia, meglitinides should not be taken if a meal is skipped. Think of the axiom "If you skip a meal, you skip a dose."
Meglitinides are typically only used in patients with T2DM who have sulfa allergies, irregular meal schedules, or late postprandial hypoglycemia when taking a sulfonylurea.
Repaglinide [Prandin]
Repaglinide comes as an IR tablet taken three times a day with meals. It has a max dose of 4 mg PO per dose, or 16 mg per day. It's got a few relevant drug interactions. Notably, it can't be used with clopidogrel or gemfibrozil (they increase repaglinide concentrations and the risk of side effects).
Nateglinide [Starlix]
Nateglinide also comes as an IR tablet (60 mg or 120 mg) taken three times a day with meals.
Thiazolidinediones (TZDs)
TZDs include rosiglitazone and pioglitazone. They lower blood sugar by improving target cell response to insulin. TZDs do not increase insulin secretion, but they are dependent on the presence of insulin for their activity (so there's little to no risk of hypoglycemia).
TZDs work by agonizing peroxisome proliferator-activated receptor-gamma (PPAR-gamma), which is present in your liver, muscle, and fat cells.
(Quick note, don't confuse that with PPAR-alpha, which is the target of fibrates and leads to reduced triglycerides).
PPAR-gamma is also present in the renal collecting tubules, which leads to the most notable toxicity of TZDs.
When TZDs stimulate PPAR-gamma in the renal tubules, they cause sodium reabsorption and fluid retention. That fluid retention makes TZDs contraindicated in patients with NYHA Class III and IV heart failure. There's also a black box warning for TZD's ability to cause or worsen heart failure.
TZDs typically lower A1c 0.6% to 1.3%. However, it's worth noting they have a slow onset and take 4-6 weeks to see an effect.
Pioglitazone [Actos]
Pioglitazone comes in 15 mg, 30 mg, and 45 mg tablets. It is typically initiated at 15 to 30 mg PO once daily but can be increased to 45 mg daily. Patients with Class I and II heart failure should start on the lower end of 15 mg (and remember, just stay away in Class III and IV heart failure).
During therapy, monitor for adverse effects such as weight gain, edema, and any other signs or symptoms of heart failure. Pioglitazone may be taken with or without food and does not require renal adjustment.
Rosiglitazone [Avandia]
Rosiglitazone used to have an additional black box warning for risk of MI, but this was removed by the FDA in 2013. Unfortunately for the shareholders, the damage was already done, and now it is not used as frequently as pioglitazone.
Rosiglitazone is dosed in single or divided doses of 4 mg PO/day or 8 mg/day. It can be taken with or without food and there is no renal adjustment.
DPP-4 inhibitors
DPP-4 inhibitors include sitagliptin, saxagliptin, linagliptin, and alogliptin. They lower blood sugar by inhibiting the dipeptidyl peptidase 4 (DPP-4) enzyme, which increases postprandial incretin (GLP-1 and GIP) concentrations.
Incretins (glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP)) regulate glucose by increasing insulin release from pancreatic beta cells, and by decreasing glucagon secretion from pancreatic alpha cells. Since DDP-4 inhibitors stop the breakdown of incretins, they have this same effect (just in a roundabout way).
Due to their mechanism of action, DDP-4 inhibitors (and GLP-1 agonists) decrease post-prandial blood glucose more than fasting blood glucose (once again, having a low relative risk of hypoglycemia compared to sulfonylureas).
They're dosed once daily (usually) and may be taken with or without food.
DPP-4 inhibitors lower A1c 0.4% to 0.8%. They are weight neutral (i.e. they don't cause weight gain), which is a major perk for patients who have gained weight from other therapies.
All DPP-4 inhibitors (except linagliptin) require renal dose adjustment. Linagliptin is eliminated through the bile and feces, not the kidneys like its counterparts.
In the adverse effects department, DPP-4 inhibitors can cause diarrhea, abdominal pain, headache, and pancreatitis.
Sitagliptin [Januvia]
Sitagliptin is dosed 100 mg PO once daily, with or without food. As noted above, there is a renal dose adjustment. If eGFR is ≥ 30 to < 50 mL/min/1.73m2, the dose is 50 mg PO daily. If eGFR is < 30 mL/min/1.73m2, the dose is further decreased to 25 mg PO daily.
Saxagliptin [Onglyza]
Saxagliptin is dosed from 2.5 mg to 5 mg PO once daily without regard to meals. Saxagliptin tablets are film-coated and should not be split, crushed, or chewed (they must be swallowed whole), so make sure to include that info when you're counseling your patients.
If eGFR is < 45 mL/min/1.73m2 or if the patient taking strong CYP3A4/5 inhibitors (clarithromycin, protease inhibitors, azole antifungals, etc), the dose is decreased to 2.5 mg once daily.
Linagliptin [Tradjenta]
Linagliptin dosing is 5 mg PO once daily. It's the DPP-4 inhibitor of choice in patients with renal impairment since there's no renal dose adjustment.
Alogliptin [Nesina]
Alogliptin dosing is 25 mg PO once daily, with or without food. There's a dose adjustment to 12.5 mg daily for CrCl ≥ 30 to < 60 mL/min. For CrCl < 30 mL/min, the dose is further reduced to 6.25 mg once daily (if patient is on hemodialysis (HD), alogliptin can be given without regard to the timing of HD).
SGLT2 Inhibitors
SGLT2 inhibitors include canagliflozin, dapagliflozin, and empagliflozin (and new for this update, ertugliflozin and bexagliflozin!). They lower blood sugar by inhibiting the sodium-glucose cotransporter 2 (SGLT2) in the proximal renal tubules. This reduces reabsorption and increases the excretion of glucose in the urine.
Since you're basically putting food for microbes in your urine, it's common to see genitourinary infections such as UTIs, vulvovaginal candidiasis, and fungal genital infections with SGLT2 inhibitors.
Additional side effects include increased urine output (hello, osmosis!), increased thirst, increased risk of bone fractures, and increased serum potassium levels. SGLT2 inhibitors work quickly; usually showing an effect within 24 hours.
They typically lower A1c 0.7% to 1.1%. All SGLT2 inhibitors require renal dose adjustment.
In general, SGLT2 inhibitors reduce the risk of CV events and death. Empagliflozin has been shown to be superior when compared to other SGLT2 inhibitors.
It’s recommended to take these in the morning. You know, unless your patient wants to be up peeing all night. They can generally be taken with or without food.
It’s also recommended they be held a few days before surgery, as case reports have demonstrated an increased risk of euglycemic diabetic ketoacidosis (eDKA) when combined with fasting often required prior to surgery.
Canagliflozin [Invokana]
Canagliflozin was the first SGLT2 inhibitor (the OG, if you will). It is initially dosed at 100 mg PO once daily prior to the first meal of the day (no matter what renal function is).
If eGFR is ≥ 60 mL/min/1.73m2, you can increase the dose to 300 mg daily (you have to keep it at 100 mg if eGFR is between 45 and 60 mL/min/1.73m2). If eGFR drops to < 45 mL/min/1.73m2, canagliflozin should not be used.
Canagliflozin previously carried a black box warning for an increased risk of lower limb amputation (approximately 2x the risk) in patients with T2DM with CVD (or risk of CVD). This warning was removed in 2020.
Dapagliflozin [Farxiga]
Dapagliflozin is started at 5 mg PO once daily, and can be titrated up to 10 mg if needed. It is contraindicated at eGFR < 60 mL/min/1.73m2. This really limits its use, especially in an elderly patient population.
Empagliflozin [Jardiance]
Empagliflozin is initially dosed at 10 mg PO once daily, but may be increased to 25 mg once daily. Its renal adjustment doesn't show up until eGFR is < 45 mL/min/1.73m2, but it's contraindicated below that.
Ertugliflozin [Steglatro]
Ertugliflozin is started at 5 mg PO once daily, and can be titrated up to 15 mg once daily if needed. Use is not recommended when eGFR is < 45 mL/min/1.73m2.
Bexagliflozin [Brenzavvy]
Last but not least, we have bexagliflozin. Bexagliflozin is dosed at 20 mg PO once daily. Tablets should be swallowed whole, and use is not recommended when eGFR is < 30 mL/min/1.73m2.
GLP-1 Agonists
If I had a dollar for every time I heard the word “Ozempic”, I’d be a millionaire by now. Okay, that’s an exaggeration… But I’d probably at least be a thousandaire. That’s a real word, btw. Look it up.
These days, GLP-1 agonists are flying off the shelves. At one point, demand was so high that the FDA started allowing pharmacies to compound these drugs due to a national shortage (which is now resolved).
We talked about these a bit already when we covered DPP4 inhibitors. They both target incretins, but rather than preventing their breakdown, GLP-1 agonists mimic the naturally occurring hormone to regulate blood sugar and appetite.
They were originally designed and approved to treat T2DM, but have more recently been used to take advantage of one of their side effects: weight loss.
Ironically, Ozempic (the current catch-all term for GLP-1 agonists) isn’t actually approved for weight loss. The only agents that are (Wegovy, Saxenda, and Zepbound) are injectable, so we won’t be covering them in this post.
In fact, none of the GLP-1 agonists were available in oral form when this post was originally written. But it’s 2025 now (HOW?!), and we have an orally available agent: semaglutide (Rybelsus). Note this is the same active ingredient as Ozempic and Wegovy, but they all have different indications based on how they are formulated, dosed, and studied.
All of the GLP-1 agonists carry a boxed warning for increased risk of thyroid cancer, although current evidence suggests the risk is low. Still, these agents should be avoided in patients with personal or family history of thyroid cancer.
There are two different formulations of Rybelsus (R1 and R2), and they are NOT substitutable on a mg per mg basis. R1 is started at 3 mg PO once daily, and R2 is started at 1.5 mg PO once daily. Dosages may be titrated every 30 days (up to 14 mg PO once daily for R1 and 9 mg PO once daily for R2) until glycemic control goals are met.
Gastrointestinal side effects like nausea, decreased appetite, diarrhea, and constipation are common, but usually improve with continued use.
There is another GLP-1 agonist in the pipeline being developed by Eli Lily called orforglipron (try saying that three times fast). It’s currently undergoing phase 3 trials for T2DM and weight management.
Alpha-glucosidase Inhibitors
In this family we have acarbose and miglitol. These medications inhibit intestinal alpha-glucosidase, which results in slowed digestion and absorption of carbohydrates such as glucose. Note that they don't reduce absorption of carbohydrates, they just slow it.
Alpha-glucosidase inhibitors are kind of a pain to take, which means they aren't the first choice for most patients. They're dosed three times daily with the first bite of each meal. They can cause flatulence, diarrhea, and abdominal pain, but these side effects are usually worse in the beginning of therapy and tend to become less bothersome over time.
Acarbose [Precose] and Miglitol [Glyset]
These two are are basically the same. Initial dosing is 25 mg PO three times daily with the first bite of each "main" meal. Dosing may be increased at 4 to 8 week intervals based on tolerability and post-prandial glucose or A1c levels, and should be avoided if SCr is above 2 mg/dL.
Bile Acid Sequestrants (colesevelam [Welchol])
Yes, there are other bile acid sequestrants out there, but colesevelam is the only one with an indication for T2DM. Colesevelam binds bile acids in the intestinal tract, which increases hepatic bile acid production.
The mechanism of action for colesevelem in T2DM (Image)
How does that help with blood sugar? We don't really know.
But through some mechanism, colesevelam causes decreased glucose production by the liver and increased incretin levels.
Colesevelam is dosed once or twice daily and should be taken with food.
Colesevelam has the same side effects as it does when used for hyperlipidemia. Constipation, stomach upset, and hypertriglyceridemia (it's contraindicated if triglycerides (TGs) are above 500). It has no renal dose adjustment.
Bromocriptine (Cycloset)
You may be surprised to see bromocriptine listed as an oral glucose-lowering agent, since it is more commonly known as an anti-Parkinson agent. While bromocriptine is a dopamine D2 receptor agonist, it also helps to treat T2DM through an unknown mechanism of action.
The mechanism of bromocriptine in T2DM (Image)
It is postulated that bromocriptine affects circadian rhythms, which play a role in obesity and insulin resistance.
When bromocriptine is administered in the morning, it may help to reverse insulin resistance and decrease glucose production by resetting hypothalamic circadian activities that were altered by obesity.
Although bromocriptine is not often used for the treatment of T2DM, the ADA acknowledges that it may be tried in specific situations.
One important point is that only the Cycloset brand is approved for use in T2DM. The brand Parlodel should not be used here (and that could be a potential test question… Just remember: PARlodel, PARkinson’s).
When used for T2DM, it is initiated at a dose of 0.8 mg PO once daily. The dose may be increased weekly in 0.8 mg increments, up to a maximum dose of 4.8 mg per day. This is a much lower dose than the Parkinson’s dose, so be on the lookout for that if you're verifying orders.
Side effects of bromocriptine include dizziness, drowsiness, headache, constipation, and nausea. Bromocriptine is also a major substrate of CYP3A4, meaning there is a big risk for drug-drug interactions. CYP3A4 inhibitors can increase bromocriptine concentrations, worsening the likelihood/severity of side effects.
It’s also contraindicated for patients that are breastfeeding due to its lactation-suppressing effects and reports of potentially life-threatening side effects in postpartum women.
When to Initiate Therapy for Type 2 Diabetes Mellitus
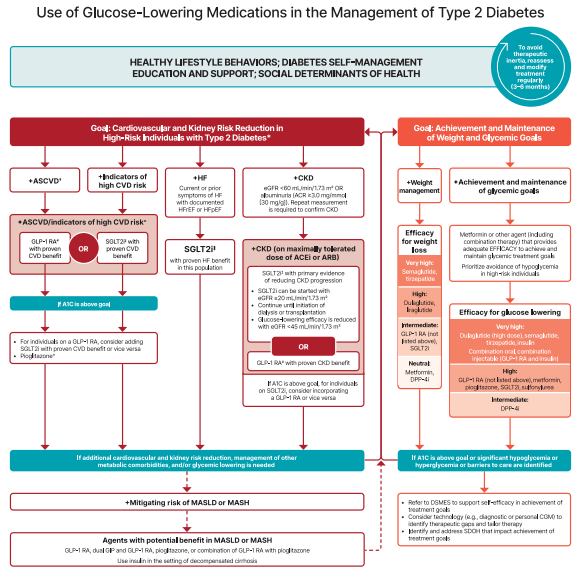
Phew! Did you get all of that? Now, you may be wondering when to start treating a patient with all the agents we just covered. Luckily, the super smart people at the ADA have created a beautiful algorithm for us. Note that it does include insulin and other injectable glucose-lowering medications that were NOT included in this article.
The screenshot is a little hard to see, so I’d recommend following the link to get the ✨full experience✨. There’s also a nifty chart summarizing key info about all the medications we covered in this article!
Final Points on Oral Glucose-Lowering Agents
DM is a common, yet complicated disease that results in significant morbidity and mortality if left untreated. It needs to be managed with a combination of lifestyle modifications and pharmacologic treatment.
Because there are so many classes of drugs used for DM (and the drugs within each class can differ widely), DM is a huge area for pharmacist opportunity. We can make quality interventions and really impact patient care with our drug expertise.
For more information on oral glucose-lowering agents and their place in therapy, check out tl;dr pharmacy’s Diabetes Cheat Sheet (seriously, it's really good and serves as a handy summary for everything this post covered and more!).