How Low Can You Go?: A Clinician's Overview of Anemia
This article has been reviewed and is up-to-date as of 09/06/2024.
Steph’s Note: Welcome back for another downright useful guest post! Sure, sometimes we like to chat about diseases and drugs that you almost never see just because they’re COOL (and when you do see them, you’ll have a baseline). But today, we’re talking about something that you probably can’t get through a single day of pharmacist-ing without encountering.
Please give a hand for Lauren Hartsell for leading our conga line under the limbo bar to figure out just how low we can go. Or at least how low your hemoglobin can go…
Lauren is a fourth-year pharmacy student at Wingate University’s School of Pharmacy (graduating in 17 days!). She will soon be starting her PGY1 residency at Novant Presbyterian Medical Center in Charlotte, North Carolina. Her long-term goals are to complete a PGY2 in cardiology, become a Board Certified Pharmacotherapy Specialist (BCPS), and return to the classroom as a professor. In the meantime, she likes to add to her Kate Spade and Lilly Pulitzer collections, watch Marvel movies, and work out.
For her debut into the tl;dr world, she has tag teamed with Justin Arnall and Chris Larck, both PharmD, BCOP, CPP, Clinical Pharmacist Coordinators. Dr. Arnall is the coordinator of Hemophilia/Hematology & Oncology Pharmacy Services at Carolinas Health Care System Specialty Pharmacy, and Dr. Larck is the coordinator of Adult Hematology/Oncology Pharmacy Services at Levine Cancer Institute (both located around Charlotte, North Carolina).
Here’s the mic, Lauren, take it away!
Hopefully this doesn’t come off insensitive, but sometimes learning a disease state is easier when you know someone who has it, right? Well, when studying anemia, you’ve got a pretty good shot at this (no pun intended).
One in four of the people reading this post right now (globally, nearly 2 billion - yes, with a “b”) have anemia, with the scales tipping slightly towards females having a higher prevalence. Why aren’t we talking about anemia more if it’s so prevalent, you ask?!
Realistically, a large percentage of you probably have never been officially diagnosed since anemia is one of the most under-diagnosed conditions in the United States.
Anemia is one of those disease states that you see on a person’s past medical history and you kinda overlook it because it’s just that common. And it just doesn’t hold the same pizazz and intrigue as, say, cystic fibrosis or hereditary angioedema…
Which is unfortunate. Because, if you actually sit and think about it, what exactly is anemia? And how do we treat it?
Don’t worry, tl;dr has your back again with this latest installment!
What is Anemia?
In its simplest definition, anemia is a state of decreased oxygen-carrying capacity that results from not making enough red blood cells (RBCs), not making them well, or making and then destroying them (hemolysis).
The term “anemia” also serves as an umbrella term for a group of diseases that are characterized by decreases in RBC volume or hemoglobin, limiting the ability to effectively move oxygen throughout the body.
How is Anemia Diagnosed?
Generally speaking, diagnosing anemia is fairly straightforward. Now, diagnosing the exact CAUSES of said anemia can get difficult. Why else would we have hematology specialists in this world?!
Lab diagnosis of single-type anemia is usually the gold standard (mixed anemias may have overlapping lab findings), but most of our anemic patients initially present to clinicians with signs and symptoms of the disease. Signs and symptoms can be (and usually are) non-specific, but there are a few that stick out like a sore thumb.
Signs and Symptoms of Anemia
Anemia can present either rapidly or over time, and the symptoms differ significantly between the two:
Acute onset
Palpitations, angina, orthostatic lightheadedness, and shortness of breath (low oxygen...makes sense)
Chronic onset
Fatigue (most common), dizziness, shortness of breath*, headache*, arrhythmias*, difficulty concentrating, and pale/cold skin [*especially with exercise]
In addition, some specific types of anemia can present in unique ways.
For example, those with iron deficiency anemia (IDA) may complain of tongue pain, smooth tongue, pica (eating non-food items such as dirt), or pagophagia (compulsively eating ice).
Does this mean that every 3rd grader who tries to eat dirt or napkins is iron-deficient? No. But, in conjunction with other symptoms, this could signal a problem.
On the other hand, vitamin B12 (cobalamin) deficiency may present more seriously with neurologic symptoms appearing before physical ones. These could include psychiatric changes (irritability, depression), numbness and paresthesia, or even vision changes.
Signs and symptoms are great and all, but lab diagnosis is the easiest way to confirm the presence of anemia. To prove our point, here are some labs… doing lab work… for lab diagnosis:
Laboratory Diagnosis of Anemia
No, not the band… (Image)
When initially evaluating a patient with suspected anemia, it’s important to look at their blood (duh...). More specifically, it’s important to obtain a complete blood count (CBC) with differential, reticulocyte index, and possibly an examination of a stool sample for occult blood.
According to the World Health Organization (WHO), a primary diagnosis of anemia can be made based on the following hemoglobin (Hgb) and hematocrit (HCT) levels:
Females: Hgb < 12 g/dL (or HCT < 36%) Males: Hgb < 13 g/dL (or HCT < 41%)
Remember, hemoglobin is the molecule that binds oxygen and is composed of heme, globin, and iron, while hematocrit is the percentage of RBCs in whole blood.
As opposed to the diagnosis of anemia, classification of anemia is usually based on a combination of lab values rather than just one single lab. Here are some of the other labs needed to classify the type of anemia and help identify the cause of anemia:
Mean corpuscular volume (MCV): the average volume of the RBCs (in femtoliters (fL)) → tells us SIZE
Mean corpuscular hemoglobin (MCH): the average Hgb content per RBC
Mean corpuscular hemoglobin concentration (MCHC): concentration of hemoglobin in the cell → Tells us COLOR
Reticulocyte count: a reticulocyte is a baby RBC → tells us about the bone marrow’s production of RBCs
Iron panel
Serum iron: the amount of iron in the blood; almost all of this is bound to transferrin
Transferrin: the protein in the blood that binds to iron and transports it into cells for storage with the help of ferritin
Transferrin saturation (TSAT): an estimation of how full transferrin already is with iron; normally ~1/3 occupied
Ferritin: the protein responsible for storing iron; usually located in the liver but also the spleen, skeletal muscle, and bone marrow; indicative of iron levels in the body
Total iron-binding capacity (TIBC): the amount of iron in the blood waiting to be bound; an indirect measure of transferrin’s capacity to bind iron; NOT a measure of transferrin itself
Folate: the amount of this vitamin in the blood
Vitamin B12 (aka cobalamin): the amount of this vitamin in the blood
The true story of Goldilocks as told by Angry Cat. (Image)
Let’s make a stretch comparison to help us remember the three main types of anemia with a reference back to our childhood and the story of Goldilocks and the Three Bears. Remember back to the beginning of the story when Goldilocks is tired (maybe fatigued from anemia and not eating enough iron in her porridge) and is trying to find a comfortable chair to rest in. One chair is too large, one is too small, and one chair is juuuust right.
With anemia, the morphologic change in RBC size can be one of the first signs we look at to determine the cause of anemia. We do this by looking at MCV. We can see microcytic cells (too small), macrocytic cells (too large) and normocytic cells (juuuust right...however in our anemia story, the “just right” chair can still break apart, so we still have some complications to prevent).
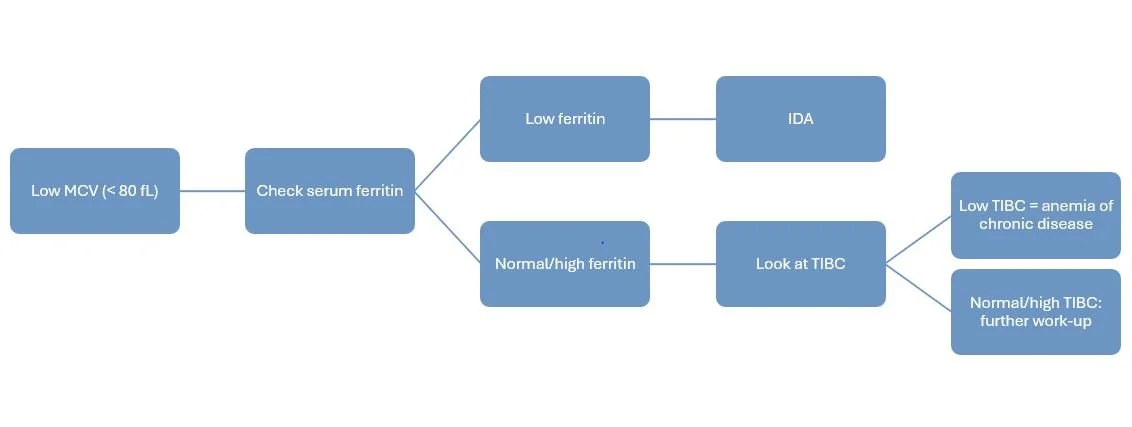
What is MICROcytic Anemia (MCV < 80 fL)?
Microcytic anemia is generally further characterized with iron studies as iron deficiency is one of the most common causes of this laboratory finding. Iron deficiency anemia (IDA) is usually secondary to inadequate diet (e.g. vegetarian/vegan diets) or increased iron requirements (e.g. pregnancy).
Quick side note: At the time of this revision, the most recent IDA guidelines are the ones published by the American Society of Hematology (ASH) in 2019. However, ASH has proposed a plan to update these guidelines. The publication date is TBD, but their goal is to have them approved by the end of 2025, so we’ll take another look at this article when those come out.
Serum ferritin is one of the best measures of iron levels and is a good next step to investigate after noticing that MCV is low. A low ferritin level (generally < 15 - 30 mcg/L) is indicative of IDA. Higher thresholds may be used for patients with certain comorbidities.
Other useful lab tests to use along with ferritin include a low serum iron level, low transferrin saturation, and/or high TIBC. Think of TIBC like seats on a bus. If the TIBC is high, there are a lot of empty seats (i.e., more capacity to bind iron). If TIBC is low, there are few available seats on the bus.
Serum ferritin and TIBC can help us differentiate between IDA and and anemia of chronic disease.
Of course, it’s not quite that easy… and there’s a “but”. There’s ALWAYS a “but”.
Unfortunately, interpreting iron studies can be tricky since ferritin is also an acute phase reactant. This means it can be elevated during periods of inflammation, infection, or trauma. Because these ferritin elevations are thought to be due to a redistribution of iron stores and acutely increased ferritin synthesis rather than an absolute increase in body iron, interpreting ferritin levels during these times may lead to a missed diagnosis of IDA. Remember when we said higher ferritin thresholds can be used for patients with certain comorbidities? This is why!
Because ferritin is largely stored in the liver, this lab can also be misleadingly elevated in cases of hepatocyte injury and subsequent release of stores. Fantastic.
So what do we do?
We can try to correlate ferritin levels with the patient’s clinical picture (was he admitted for sepsis when there’s inflammation and infection?). We can also try to correlate ferritin with other measures of inflammation, such as C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR). Are the patient’s liver function tests elevated and indicative of hepatocellular injury?
All that being said, getting a clear diagnosis of IDA using iron studies during periods of acute illness can be super difficult!
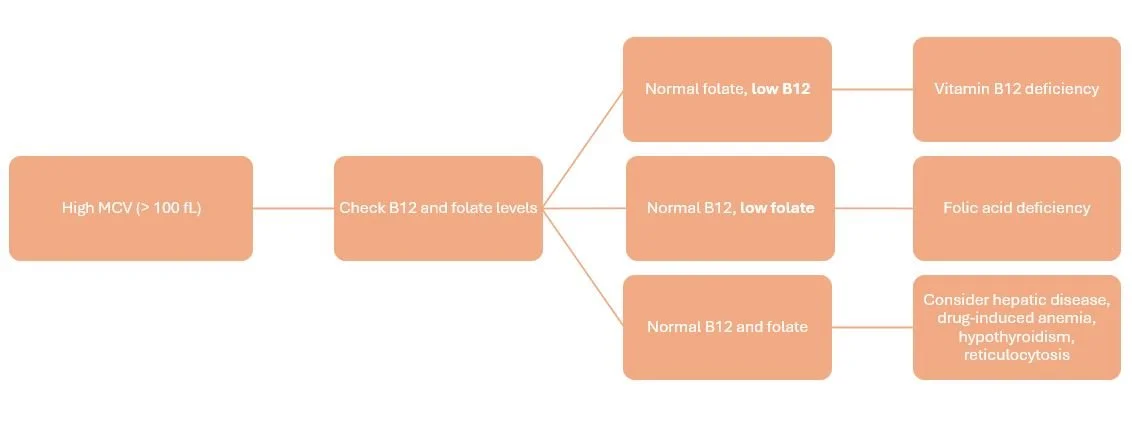
What is MACROcytic Anemia (MCV > 100 fL)?
When our blood cells are too large, low vitamin B12 (less than 200 pg/mL) or low folate levels (less than 4 ng/mL) are the most common causes.
Log this picture away! It’s the process of vitamin B12’s absorption, which is made possible by its wingman, intrinsic factor, and its Uber driver, transcobalamin. (Image)
If B12 and intrinsic factor were the most adorable mice in the world. Of course, I guess that makes the bottom mouse the receiving ileum? (Image)
If a patient has a low vitamin B12 level, it may be due to loss or inactivation of intrinsic factor. Intrinsic factor is a protein secreted by the gastric parietal cells, and it is required for absorption of B12 in the ileum. It’s B12’s wingman!
Once absorbed into the ileal cells, transcobalamin takes over for intrinsic factor and ferries the vitamin B12 to its next destination (mostly the liver for storage). AKA transcobalamin is B12’s own personal Uber driver.
The historical gold standard for determining abnormalities in B12 absorption was the (radioactive) Schilling test. However, this test isn’t widely available in the United States anymore. Now, we rely more heavily on a series of lab tests in order to detect and confirm B12 deficiency.
The lab tests used are dependent on the key fact that vitamin B12 is involved in the conversion of methylmalonic acid (MMA) to succinylcholine coenzyme A (whatever that’s for…). But if a patient has an unclear initial lab picture consisting of macrocytosis (high MCV) and low/normal B12 levels, a high MMA is a good next step to see if his B12 level is up to snuff for doing its metabolic job. A high MMA would indicate B12 deficiency, which is why it can be used as a confirmatory test.
Common causes of low vitamin B12 include nutritional deficits (i.e. need to eat fish, meat, and dairy!), gastric bypass (i.e. lost geography for intrinsic factor secretion), and decreased gastric acid production (i.e. use of PPIs).
But there is another, more insidious form of B12 deficiency. It’s a severe form of B12 deficiency called pernicious anemia, which can be one result of an auto-immune gastritis. In some people, the body produces intrinsic factor antibodies (anti-IF), which can damage parietal cells and/or block the action of intrinsic factor. If pernicious anemia is suspected, patients will be tested for the presence of anti-IF and elevated gastrin levels.
Now that we went through all the crazy B12 info, on to folate.
Macrocytic anemia due to folate deficiency is way less exciting. Basically, patients are nutritionally deficient and need folate. Whoop-de-freakin’-doo.
Now, if your patient has a macrocytosis but both B12 and folate levels are normal, it may be necessary to rule out drug-induced anemia, hepatic disease, thyroid disease, or reticulocytosis.
Of note, liver disease can cause falsely elevated B12 levels (the liver’s not processing normally so more stays floating around in the blood). Remember that “but” we talked about in the microcytic section? There it is for the macrocytic section. You’re welcome.
What is NORMOcytic Anemia (MCV 80-100 fL)?
When MCV is normal (normocytic cells), the first step is to rule out RBC destruction or blood loss. This is where reticulocyte count comes in.
Reticulocytes are immature RBCs. They are usually made more rapidly following blood loss or RBC destruction/hemolysis in order to try to replenish the missing RBCs and improve oxygen delivery. If reticulocytes are high, you should consider acute blood loss (which should hopefully show itself for the nasty bleed it is - but doesn’t always). You should also consider drug-induced hemolysis (i.e., sulfonamides, dapsone, ribavirin).
If reticulocytes are low, WBC and platelets can help to rule out the presence of bone marrow failure (leukemia, aplastic anemia). If WBC and platelets are normal/high, suspect chronic infection/inflammation, malignancy, or CKD as the cause.
How to Correct Iron Deficiency Anemia
Treatment of IDA focuses simply on replenishing iron stores. However, this is not always an easy task! There are many iron products available with varying degrees of iron content to complicate the matter.
Oral Iron Supplementation
Historically, it was recommended to provide between 100 and 200 mg of elemental iron daily, preferably divided into two to three separate doses to improve absorption. However, there’s some more recent information regarding iron supplementation’s effects on hepcidin that are starting to change the game.
So what is hepcidin, and why on earth are we introducing yet ANOTHER obscurely named molecule in this post?? Wasn’t intrinsic factor ENOUGH?!?
Hepcidin is the main regulator of iron balance in humans. It’s a protein that actually works to INHIBIT iron transport from intestinal cells into circulating blood. As such, its levels are inversely related to iron bioavailability, meaning higher hepcidin levels correlate with lower iron bioavailability. What’s the big deal?
Well, the new thought is that if we give multiple doses of supplemental iron throughout the day as previously recommended, we may actually be creating an environment for diminished iron absorption!
Using this information, many providers have backed down to daily or even every other day dosing of oral iron supplements. The optimal dosing strategy hasn’t quite been elucidated, but we do seem to be on board with nixing BID or TID iron dosing.
Some side effects of long-term oral iron use include: GI upset, constipation, metallic taste, and dark/green stools.
Interestingly, slow-release iron is not absorbed as efficiently as regular-release iron since absorption is delayed until the iron product reaches the small intestines. Key point: an acidic environment is required for maximal iron absorption (and the small intestines are pretty basic...or alkaline). This is also why patients taking PPIs or those with gastric bypass (less time exposed to an acidic environment) may have impaired iron absorption.
There are many oral iron products available, but the big three are:
ferrous sulfate (20% elemental iron)
ferrous gluconate (12% elemental iron)
ferrous fumarate (33% elemental iron)
Oral iron dosing is based on the elemental iron content, so a typical 325 mg dose of ferrous sulfate is actually only 65 mg of elemental iron (i.e. 325 x 20% = 65).
Intravenous (IV) Iron Supplementation
So when does my patient need to get iron supplementation from an IV?
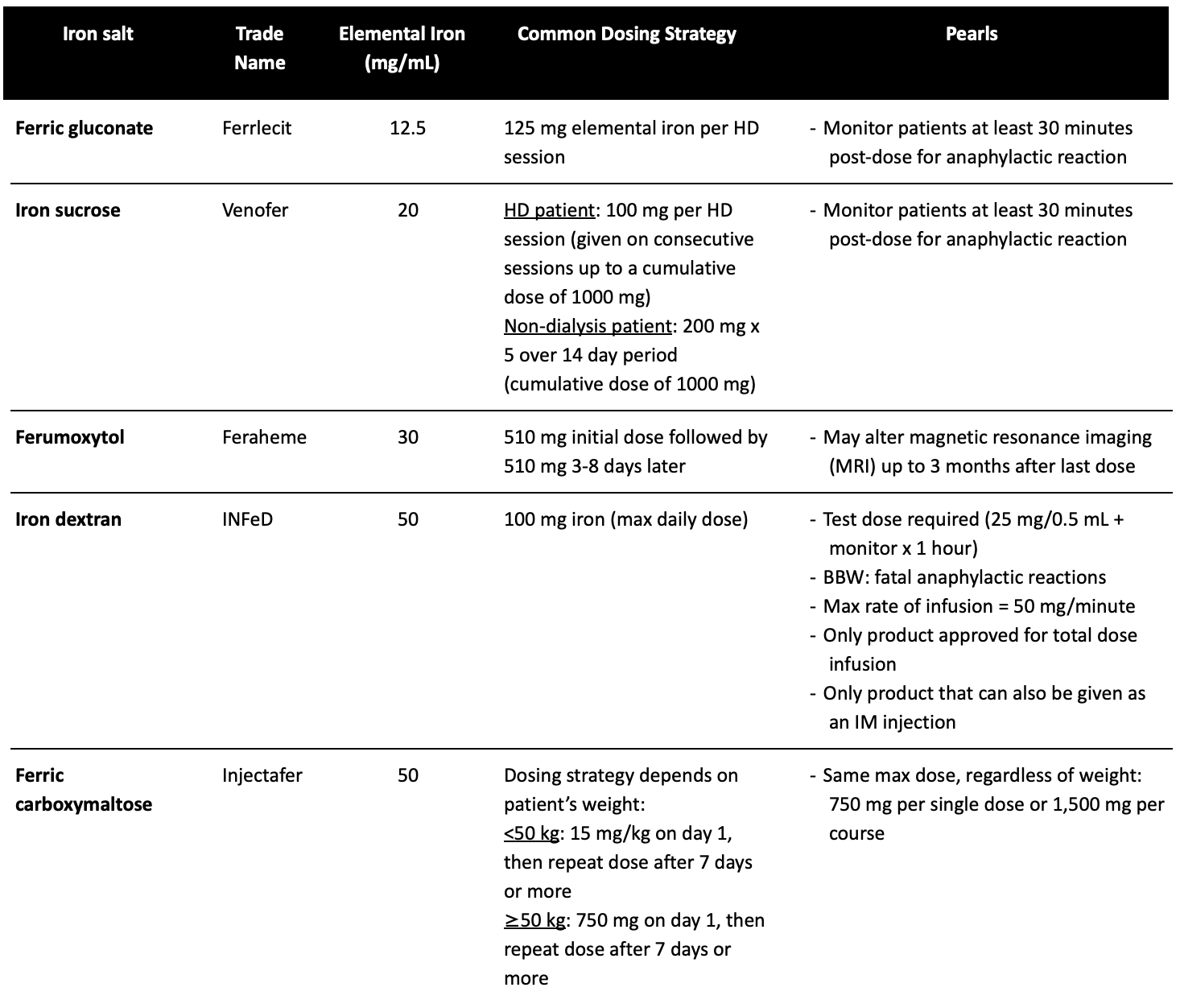
The indications for parenteral iron include: intolerance to oral therapy, malabsorption (consider in gastric bypass or inflammatory bowel disease), and nonadherence. We have listed some of the quick and dirty clinical pearls on the IV iron products as well as a summary chart of the most common IV iron products that may come in handy.
Pearls for Parenteral Iron
Patients with active infection should NOT receive IV iron (possible risk of feeding iron-dependent bacteria)
Necessary to use in CKD patients on hemodialysis, those on erythropoietin stimulating agents (ESAs), or unstable patients with absorption problems
Avoid in first trimester of pregnancy
Hypersensitivity reactions are common! These can be immediate or delayed.
Immediate (within minutes of starting infusion)
Malaise, urticaria, nausea, diaphoresis, headache
Anaphylaxis
Delayed (within 1-2 days after infusion)
Myalgias, arthralgias, fever, flu-like symptoms
How to Calculate Doses of Parenteral Iron
There are several different equations to calculate a patient’s estimated iron deficit (aka how much you need to replete). These equations include the Ganzoni equation, the modified Ganzoni equation, as well as a few others. They’re all fairly similar in that they use a patient’s weight and hemoglobin, as well as a target hemoglobin, to determine how much iron to give.
Because there are so many, we won’t list them here. The best rule of thumb = check your chosen iron product’s package insert to see which formula is recommended for that particular product.
About those products…
Note that the listed dosing strategies are for ADULT patients. All information can be found in the respective package inserts.
How to Correct Vitamin B12 Anemia
Treatment of this type of anemia is more straightforward than IDA. Basically, it just takes giving patients some B12. How much depends on how clinically severe the deficiency is and what route we’re using.
Subcutaneous or intramuscular injections may be used, but studies have shown that high doses (1-2 g) of oral supplementation may be just as effective. Nasal sprays have also been approved, although all brand name products (Nasacobal, Calomist) have been discontinued. It appears Lupin still manufactures a generic version.
How to Correct Anemia Due to Folate Deficiency
Guess what? This anemia’s even MORE straightforward than the previous two types! #win!
Choose your own adventure for oral or intravenous repletion, but beyond that, it’s just 1-5 mg daily x 1-4 months (longer for chronic conditions such as alcoholism or hemodialysis).
We are SO NOT following her!!! Far… (Image)
Lastly, we are not going to venture very far down this rabbit hole (maybe in another post!), but we will give an overview of the most important points for pharmacists on erythropoietin stimulating agents (ESAs).
Erythropoietin Stimulating Agents: The Basics
ESAs do exactly what it sounds like they do. They stimulate erythropoietin. (Dur…)
Oh wait, actually not dur. Erythropo-what?
Erythropoietin (EPO) is a hormone produced by the kidneys. It’s stimulated by the presence of low oxygen via a feedback loop, and it’s responsible for jumpstarting the production of RBCs.
So, kicking this hormone into gear is a good thing, but dosing and monitoring these agents requires some work. And they’re certainly not benign medications.
Who gets these non-benign meds?
We use ESAs in patients who have anemia associated with chronic kidney disease (CKD). Remember, EPO is made primarily in the kidneys...makes sense we would need to give a little extra help in patients whose kidneys aren’t working at full capacity.
We also use ESAs in anemia associated with chemotherapy, in patients with HIV treated with zidovudine, or in those at risk for perioperative blood loss.
The DOs and DONTs of Erythropoietin Stimulating Agents
DO use the lowest dose possible to achieve desired result
DO reduce dose if Hgb rises rapidly (> 1 g/dL rise in a 2-week period) → reduce by > 25%
DO increase the dose by 25% if Hgb has not increased by at least 1 g/dL after 4 weeks
DO recommend the IV route for hemodialysis patients (package inserts recommend this, although there may be some dose-reduction and cost-saving benefits of utilizing the subcutaneous route in HD patients)
DO ensure your patient’s iron is sufficient before initiating ESAs
DON’T use when Hgb > 11 g/dL (increased risk of embolic events)
DON’T increase dose more than once every 4 weeks (decrease can be more frequent)
DON’T increase dose if no response after 12 weeks escalation period (may even discontinue therapy for unresponsiveness)
How to Dose Erythropoietin Stimulating Agents
Epoetin alfa (Epogen®, Procrit®) and biosimilar epoetin alfa-epbx (Retacrit®)
Patients with CKD (+/- dialysis)
Adults: 50-100 units/kg 3x/week IV/SQ
Pediatric patients (1 month & up): 50 units/kg 3x/week IV/SQ
Zidovudine-treated patients with HIV
100 units/kg IV/SQ 3x/week
Chemotherapy patients
Adults: 150 units/kg 3x/week SQ or 40,000 units SQ weekly until completion of chemotherapy
Pediatric patients (5-18 y/o): 600 units/kg IV weekly until completion of chemotherapy
Surgery patients
300 units/kg/day SQ x 15 days total (administered daily x 10 days before surgery, the day of surgery, and for 4 days after surgery) OR
600 units/kg SQ x 4 doses (administered 21 days, 14 days, and 7 days prior to surgery plus the day of surgery)
Darbepoeitin alfa (Aranesp®)
Patients with CKD
Adults on dialysis: 0.45 mcg/kg IV/SQ weekly OR 0.75 mcg/kg IV/SQ every 2 weeks
Adults not on dialysis: 0.45 mcg/kg IV/SQ every 4 weeks
Pediatric patients: 0.45 mcg/kg IV/SQ weekly (patients not on dialysis can also be given 0.75 mcg/kg every 2 weeks)
Chemotherapy patients
2.25 mcg/kg SQ weekly or 500 mcg SQ every 3 weeks
Notice the much longer dosing interval on darbepoeitin compared to erythropoietin?!? VERY IMPORTANT!
Another ESA (methoxy polyethylene glycol-epoetin beta (Mircera®)) exists, but it’s expensive and has limited indications, so it’s not really used in clinical practice.
Hopefully this post at least gets your feet wet with how to recognize and diagnose anemias, the different deficiencies that can produce symptoms, and how to treat these patients. Now that you’ve read this, the next time you’re investigating your oh-so-sexy rare diagnosis patient, you can also pay attention to that low hemoglobin!