A Pharmacist's Fact Versus Fiction: Antibiotic Edition – Part I
Steph’s Note: I was going to write an intro here for our favorite critical care guru, Dr. Josef Nissan, but Joe wrote his own note. (And if you don’t know Joe yet, you really should check out his body of work on our site, most recently here with cannabinoid hyperemesis syndrome.) So why reinvent the wheel when he’s already done such a great job with it?!
Joe’s Note: Let’s face it. We’ve all been taught flawed information throughout our career that we later found out to be untrue. Doesn’t ring a bell?
I know. Tom Hanks can’t believe it either. (Image)
Hm, what about telling patients to NEVER drink alcohol while taking metronidazole. Come on, you know you’ve done it. I know I have. You may still be doing it now. Well, there’s conflicting data that may prove the “disulfiram-like reaction” we counsel patients on may be more of a myth. If you don’t believe me, read this or maybe this.
Anyway, I recently realized just how many antibiotic myths circulate through our medical practices. These are myths that pharmacists and practitioners teach that are just flat out incorrect. So, I decided to create a two-part series called “fact or fiction” to help debunk some of the most common antibiotic myths. Some are more controversial than others, but nonetheless, I will include primary literature to back up my points.
Hopefully, by the end of this series, you can be confident in deciphering fact from fiction.
Alright, let’s dive right in.
Fact or Fiction #1: Cefdinir has low urine penetration and should NEVER be used to treat uncomplicated cystitis.
Gotta get more flair. Or, in pharmacy world, some critical thinking. (Image)
Don’t be what I call a “Lexicomp Pharmacist.” How do I define a Lexicomp pharmacist? Well, a Lexicomp pharmacist is an individual that solely uses Lexicomp as their main form of clinical evidence, refuses to look at other primary literature, and never uses their own clinical judgment. These pharmacists have a strong belief that “if Lexicomp says it, then it must always be true”.
Let us be Lexicomp pharmacists for just a second.
Go to lexicomp.com and search “cefdinir.” Now go ahead and hit the “Pharmacology & Pharmacokinetics” tab. Scroll down to “Excretion” where it says, “Primarily urine (~12% to 18% as unchanged drug).” Surely if 12-18% remains unchanged in the urine, then cefdinir clearly doesn’t have strong urinary penetration and should NEVER be used to treat urinary tract infections. Right?
On paper, it appears that way. But let’s dig into some primary literature.
The first study published by Lloyd et al. looked at treatment failure rates between cefdinir and cephalexin for the treatment of urinary tract infections. Treatment failure at 7 days occurred in 11.6% of patients in the cefdinir group and 8.3% of patients in the cephalexin group (P=0.389). Treatment failure at 14 days was higher for cefdinir at 20.7% than for cephalexin at 11.8%, but this difference was NOT statistically significant (P=0.053).
In addition, there were no differences in the rate of treatment failure in subgroup analyses of uncomplicated or complicated UTIs. Overall, the authors concluded that cefdinir and cephalexin have comparable efficacy for the treatment of lower UTIs.
Let’s pause and talk about statistical versus clinical significance for a second. Treatment failure at 14 days was 20.7% in the cefdinir group compared to 11.8% in the cephalexin group. While it wasn’t statistically significant, that’s pretty darn clinically significant to me. Almost double the patient population. So, what does that mean? Should we avoid cefdinir for all UTIs? Not necessarily.
Cefdinir can be used to treat uncomplicated cystitis. Would I use it to treat pyelonephritis or a complicated UTI? Personally, no. Is it the first drug I reach for when treating uncomplicated cystitis? Not really. But can it be used to treat uncomplicated cystitis? Most definitely.
tl;dr Summary: Despite having lower urinary penetration compared to other first-line agents, cefdinir has been shown to be effective in treating uncomplicated cystitis.
Fact or Fiction #2: Sulfamethoxazole-trimethoprim has poor streptococcal coverage.
Do you want to know what makes me lose my marbles? When providers start patients on dual cephalexin and sulfamethoxazole-trimethoprim (Bactrim) therapy for the empiric treatment of skin/soft tissue infections because “Bactrim doesn’t have good strep coverage”.
Ron Burgundy knows about the fake news surrounding Bactrim. (Image)
You know what I have to say to that nonsense? FAKE NEWS. Let me tell you why.
Back in ancient days (tbh, I have no idea when), laboratories used to primarily use thymidine-containing agar media. Well, it turns out that Streptococcus pyogenes (Group A Strep) can use an exogenous source of thymidine when biosynthesis is blocked by Bactrim, therefore giving the false impression of resistance. However, when tested appropriately by the recommended thymidine-depleted media, all S. pyogenes isolates in North America are susceptible to Bactrim with an MIC ≤ 0.12 ug/mL.
In fact, further support from this randomized trial indicated a rate of cure of uncomplicated SSTI using Bactrim to be 88.2%. If you still don’t believe me, take a look here or even here. There is just so much literature debunking this myth. So please don’t be that one clinician that starts dual beta-lactam and Bactrim therapy for the treatment of every mild skin/soft tissue infection.
tl;dr Summary: Sulfamethoxazole-trimethoprim (Bactrim) provides adequate staphylococcal & streptococcal coverage and may be used as monotherapy for the treatment of uncomplicated skin and soft tissue infections.
Fact or Fiction #3: Aminopenicillins should NOT be used to treat uncomplicated urinary tract infections caused by Vancomycin-Resistant Enterococcus species (VRE).
This one’s my personal favorite. Very rarely do I find someone that knows this clinical pearl, and it never fails to mesmerize providers. Let’s start with a small case to help us debunk this myth.
Patient JJ comes into your hospital for concerns of a worsening UTI. After further examination, the patient is diagnosed with an uncomplicated UTI, and the provider would like to start empiric antibiotics to treat the urine culture collected from an urgent care a few days ago. The culture is as shown:
Clearly, this is Vancomycin-Resistant Enterococcus (VRE). Therefore, you’re going to recommend starting daptomycin or linezolid, right? Those are generally the treatments of choice for VRE infections. However, those may NOT be the best option for this specific case.
So then, what should we start? An aminopenicillin such as amoxicillin or ampicillin. Wait, hold on. Looking at my culture, it clearly shows that this species is resistant to ampicillin. So why would I ever start an aminopenicillin? Well, let’s discuss some background information to help us better understand this.
Based on the current Clinical and Laboratory Standards, Enterococcus species with a minimal inhibitory concentration (MIC) ≥16 ug/mL are considered ampicillin resistant. However, microbiology laboratories use the same breakpoint regardless of the site of infection. Because aminopenicillins are cleared through the kidneys, we can achieve much higher concentrations in the urine than in the bloodstream. In fact, this study showed an average urine concentration of 1100 ug/mL collected over 6 hours after just a single dose of oral amoxicillin 500 mg.
As you probably remember, penicillin antibiotics display time-dependent killing. This means that an optimal response will occur if the urine drug concentration is above the MIC for at least 50% of the dosing interval. Given the high urinary concentration of aminopenicillins, we can reasonably conclude that therapeutic doses of aminopenicillins will remain above the MIC (≥16 ug/mL) and are therefore effective in treating ampicillin-resistant enterococcus isolated in lower UTIs.
To prove my point, this study published by Cole et al. measured the outcomes of aminopenicillin therapy for vancomycin-resistant enterococcal UTIs. A total of 316 urinary isolates were screened, and 61 patients with symptomatic UTIs were included. 20 (35%) of the 57 isolates were ampicillin susceptible. 31 patients received an aminopenicillin and 30 received a non-beta-lactam. Within the aminopenicillin group, the most common agent selected for definitive therapy was amoxicillin, followed by IV ampicillin, ampicillin-sulbactam, and amoxicillin-clavulanate. In the non-beta-lactam group, the most common agent selected for definitive therapy was linezolid, followed by daptomycin and fosfomycin.
The rate of clinical cure was 83.9% patients in the aminopenicillin group and 73.3% in the non-beta-lactam group. Clinical cure with aminopenicillin therapy was observed in 84% of all cases and in 86% of patients with ampicillin-resistant isolates, with no statistical difference detected between results for those treated with non-beta-lactams. As a result, the author concluded that the aminopenicillins achieve sufficient urinary concentrations to eradicate VRE and therefore may be a viable option for treating VRE UTI, regardless of the organism’s ampicillin susceptibility.
tl;dr Summary: Aminopenicillins achieve high urinary concentrations and can be used to eradicate VRE in uncomplicated cystitis, regardless of the organism’s ampicillin susceptibility.
Fact or Fiction #4: Intravaginal metronidazole can be used to treat Trichomonas vaginalis.
This is a common provider question. “Patient says she can’t tolerate oral metronidazole. Can I give her intravaginal metronidazole gel to empirically treat for trichomoniasis?” The answer is always NO.
But wait. Don’t we use metronidazole gel to treat bacterial vaginosis (BV)?
Yes, it can be used to treat BV but NOT Trichomonas vaginalis. Why, you ask?
According to the Centers for Disease Control and Prevention (CDC), metronidazole gel does NOT reach therapeutic levels in the urethra and paravaginal glands and is NOT recommended for the treatment of trichomoniasis.
Specifically, this pilot study conducted by DuBouchet et al. compares the efficacy of metronidazole vaginal gel versus oral metronidazole for the treatment of Trichomonas vaginalis. Using culture for the test of cure, trichomonas infection was eliminated in all 15 women treated with oral metronidazole, and only 7 of 16 (44%) women treated with intravaginal metronidazole. Therefore, this study showed that 0.75% metronidazole vaginal gel is NOT effective as a single agent for the treatment of trichomoniasis.
tl;dr Summary: Intravaginal metronidazole gel does NOT reach therapeutic levels in the urethra and paravaginal glands and is therefore NOT effective for the treatment of Trichomonas vaginalis.
Fact or Fiction #5: Avoid all beta-lactams in patients with a documented penicillin allergy.
Why would you want to give this sweet grandma levofloxacin? WHY, I ask you!?!? TELL MEEEEE. (Image)
This is another one of those myths that just really irks the heck out of me. I can’t stand when a 100-year-old with CKD is started on IV levofloxacin for community-acquired pneumonia because she has a documented “rash” to penicillin. If you avoid all beta-lactams because a patient has a mild allergy/intolerance, then we need to talk. Don’t be that pharmacist.
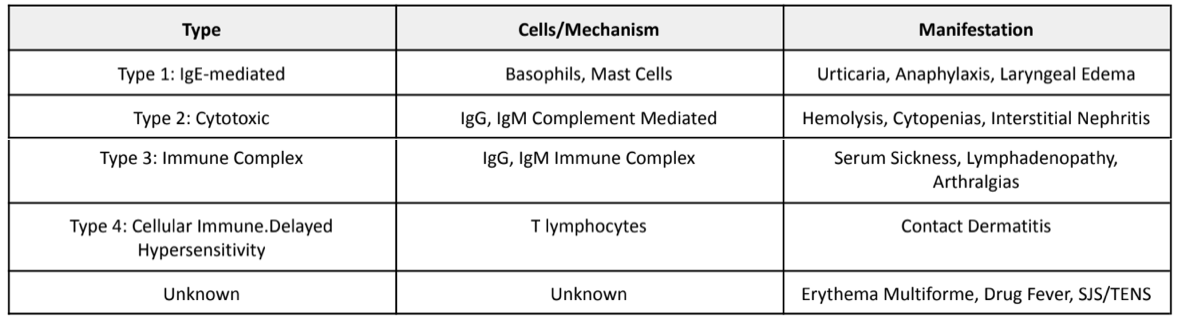
Let’s talk about some background information before we debunk this myth. Ever heard of the Gell-Coombs Classification of Hypersensitivity Reactions? If not, take a look below:
Now that we’vre reviewed the different types of hypersensitivity, let’s discuss cross-reactivity. Cross-reactivity is when drugs of the same class exhibit nearly identical toxic profiles. Meaning if patient Z is allergic to drug X, then patient Z is predisposed to developing an allergic reaction to any other drug that shares a similar chemical structure to drug X.
As we know, the beta-lactam drug class is composed of penicillins, cephalosporins, carbapenems, and aztreonam. Some of these agents share similar chemical structures which may predispose patients to cross-reactivity reactions. So, if a patient is allergic to amoxicillin, should we automatically just rule out all other beta-lactams to avoid cross-reactivity? Please don’t.
Let’s go over some very important points to always remember when assessing for beta-lactam cross-reactivity:
Cross-reactivity is ONLY associated with Type 1 IgE-mediated reactions. Cross-reactivity is NOT related to Type 2, 3, or 4 reactions. So, if a patient experiences hemolysis to penicillin (a Type 2 hypersensitivity reaction), you can safely try any other beta-lactam with no concern for cross-reactivity. Again, cross-reactivity is only associated with Type 1 IgE-mediated reactions.
Cross-reactivity is associated with similarities in R1 and R2 side chains in beta-lactams.
Almost all occur in those with similar or identical R1 side chains.
Cross-reactivity between penicillins and cephalosporins is <2%.
Cross-reactivity between penicillins and carbapenems is <1%.
Cefazolin has a unique R1 side chain that is NOT similar to any other beta-lactam.
Aztreonam can be safely used in patients allergic to all beta-lactams EXCEPT ceftazidime or cefiderocol.
That’s right folks. Cross-reactivity between penicillin and cephalosporins/carbapenems is <2%. So next time you start a 103-year-old on levofloxacin because of a “rash” allergy to penicillin, remember that I am going to haunt you.
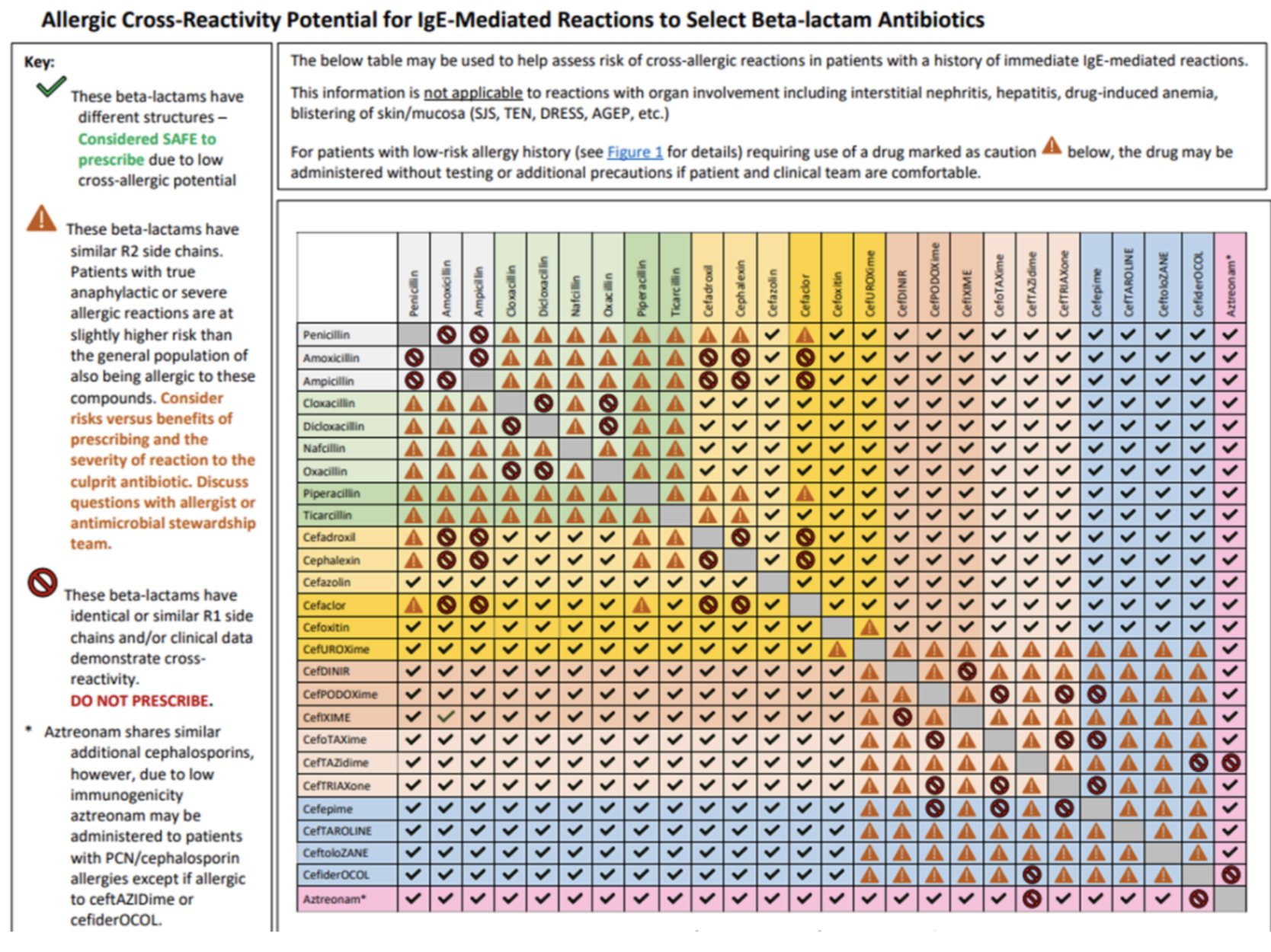
If you’re ever stuck trying to figure out which beta-lactams share similar side chains, use this table below 😊
Pop quiz time! This will help us make sure we understand how to use the above table.
Question 1: Patient has a documented allergy to amoxicillin (rash). Can the provider safely use cephalexin?
a) Yes
b) No
Answer: (B); For starters, rash is an Type 1 hypersensitivity reaction meaning cross-reactivity can occur. Now, using the table, you see that amoxicillin and cephalexin share identical/similar R1 side chains. Therefore, there IS a risk for cross-reactivity and cephalexin should NOT be used in patients with a documented allergy to amoxicillin.
Question 2: Patient has a documented allergy to penicillin (itching). Can the provider safely use ceftriaxone?
a) Yes
b) No
Answer: (A); Using the table, you see that penicillin and ceftriaxone have different structures and are considered SAFE to prescribe due to low cross-allergic potential.
Pretty self-explanatory, right?
tl;dr Summary: The cross-reactivity between penicillin and cephalosporins/carbapenems is <2%. Using the table above, many patients with documented penicillin allergies can safely tolerate numerous cephalosporins and carbapenems.
The tl;dr Summary of Fact Vs Fiction Part 1
If you skipped all my hard work above, then here’s a summary of all the myths that I debunked today:
Despite having lower urinary penetration compared to other first-line agents, cefdinir has been shown to be effective in treating uncomplicated cystitis.
Sulfamethoxazole-trimethoprim (Bactrim) provides adequate staphylococcal & streptococcal coverage and may be used as monotherapy for the treatment of uncomplicated skin and soft tissue infections.
Aminopenicillins achieve high urinary concentrations and can be used to eradicate VRE in uncomplicated cystitis, regardless of the organism’s ampicillin susceptibility.
Intravaginal metronidazole gel does NOT reach therapeutic levels in the urethra and paravaginal glands and is therefore NOT effective for the treatment of Trichomonas vaginalis.
The cross-reactivity between penicillin and cephalosporins/carbapenems is <2%. Using the table above, many patients with documented penicillin allergies can safely tolerate numerous cephalosporins and carbapenems.
If you liked this post, then make sure you stick around for part 2 of this series! In the second part of our fact or fiction series, we will debunk some more antibiotic myths such as:
Avoiding doxycycline use in pediatric patients <8 years
Nitrofurantoin use in patients with a CrCl <60 mL/min
And more!!
Until then, peace and blessings my friends :)