A Pharmacist's Quick Introduction to the Common Terminology Criteria for Adverse Events (CTCAE) Grading System
Steph’s Note: This week, we’re taking a break from the hard-hitting pharmacotherapy posts and going a little bigger picture. Maybe not a 10,000 foot view since today’s post is still super relevant to every day clinical practice, but it’s a slightly lighter load of a topic. You’re welcome as you might be getting back into the swing of studying or prepping residency applications!
Teaching us today is Dr. Jenny Hoang. Jenny is a 2024 PharmD graduate from the University of Texas at Austin. Hook 'em! Jenny is an oncology pharmacist at Texas Oncology, and she is passionate about emotional support and innovation in the cancer world. In her free time, she is even more passionate about trying every dessert on the menu and spending time with friends and family.
(Image)
Grading systems. It seems like we don’t get away from them even after schooling. That is especially true in the cancer setting. While the many drugs we use in oncology have amazing benefits, they also come with nasty side effects. And with the cancer world constantly adding new drugs to the arsenal, it is especially important to have a system that consistently defines the severity of adverse reactions in a standardized way, whether in clinical practice, research and development, or clinical trials.
As a result, today we are going to do a quick overview of the Common Terminology Criteria for Adverse Events (CTCAE).
Personally speaking, as a pharmacist fresh out of school, I can honestly tell you that my first few weeks in oncology made me sweat. Talk about imposter syndrome when they asked ME to verify chemotherapy. And based on what? Labs. So. Many. Labs.
My first impression was that I was looking at a foreign language.
But lucky for you, we have the handy dandy CTCAE to help us determine our course of treatment. So let’s dive into it.
What is the CTCAE Grading System?
Created by the National Cancer Institute (NCI), the CTCAE scale was designed to create a globally recognized system for grading and defining adverse events experienced by the patients in cancer drug clinical trials. Why is this important?
Even Stan wants adverse events to be graded in a standardized manner. (Image)
Do you remember how in college everyone would fight over having a specific professor because they were notorious for giving out a higher percentage of As according to Rate My Professor? It was unfair, right? That was because you were all getting graded on different scales. So even if you performed better on your test, if you were graded harder, you wouldn’t get as high of a GPA as Joe Bob cruising through popular Dr. Smith’s class.
Given this line of logic, this is why it is beneficial to have cancer drugs graded for their adverse events on the same standard scale. You can probably imagine how out of hand things could get if there was a subjective ability to rate adverse reactions reported during clinical trials.
Not only that, but having a standardized scale allows us to also have a reference for what to do in clinical situations. This allows everyone to be on the same page versus leaving all decisions to the provider’s individual discretion. But we’ll get to that soon.
Sold yet? I hope so, but first, let’s review some quick terminology.
What is an Adverse Event?
Defined by the NCI, an adverse event is any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medical treatment or procedure that may or may not be considered related to the medical treatment or procedure.
When you’re reading the supplementary index of a clinical trial and realize they had to include the patient’s fall with nosebleed in the minor bleeding category of the drug data… (Image)
To me, that’s Merriam-Webster’s way of saying: anything that could go wrong will be accounted for. And like we mentioned earlier, this is a pro as we want to grade all cancer drugs going through clinical trials rigorously and fairly. It can become a con, however, when you notice the phrasing “may or may NOT be related to the drug in question.” This means, if a patient trips and breaks their nose, the bleeding or potential consequent hospital visit may be summed up into the drug adverse event data.
With all of that in mind now, we can jump into the grading itself.
What are the CTCAE Categories?
Grades refer to the severity of an adverse event and range from 1 - 5 with 5 being the most severe. It goes like this:
Grade 1 - asymptomatic or mild symptoms, clinical observation only, no intervention needed
Grade 2 - mild to moderate symptoms, with maybe some local or noninvasive intervention
Grade 3 - severe and medically significant but not immediately life-threatening
Grade 4 - life-threatening consequences and urgent intervention is needed
Grade 5 - death
Furthermore, each System Organ Class (individually categorized by anatomical or physiological system in the body, e.g., gastrointestinal, vascular, immune, etc) has its own grading scale to describe the severity of an adverse effect found in that part of the body.
What does all of this mean for us as clinicians?
We can get the obvious out of the way and say if we see Grade 5…no bueno.
But for me, I often found Grades 2 and 3 a bit of a gray area. Grade 2 might not mean much for a generally healthier patient, but what about an older one with multiple comorbities? Or what if a patient consistently has Grade 3 lab results? Do we hold the line and say no more treatment, thereby risking the consequences of not having their chemotherapy?
Those questions are, again, exactly why we need a standardized scale such as the CTCAE. When things get muddy, it is a good thing to have a reference for how to proceed with treatment. As a result, the CTCAE grading scale is a great reference when reviewing a patient’s labs and determining whether to proceed with, adjust, or hold treatment.
Key Examples of How to Use the CTCAE Grading System
Now that we have established the basics, we can go over a few common ways I’ve seen this grading scale used in practice.
This urinary scale is used often for the drug bevacizumab. For quick reference, this is an antibody drug that binds to the protein VEGF, which prevents new blood circulation to a tumor. However, a major side effect is that protein can drastically accumulate and cause renal damage. So, we should test bevacizumab patients’ urine for protein content to decide whether to continue to treat or not with this medication.
It is usually recommended to hold for proteinuria ≥2 g in a 24 hour period and to discontinue bevacizumab for nephrotic syndrome. Consequently, if you were to see Grade 2+ urine protein on the lab results, you may want to proceed with caution, and if it’s a Grade 3+, then hold and hydrate.
Ok, now example #2.
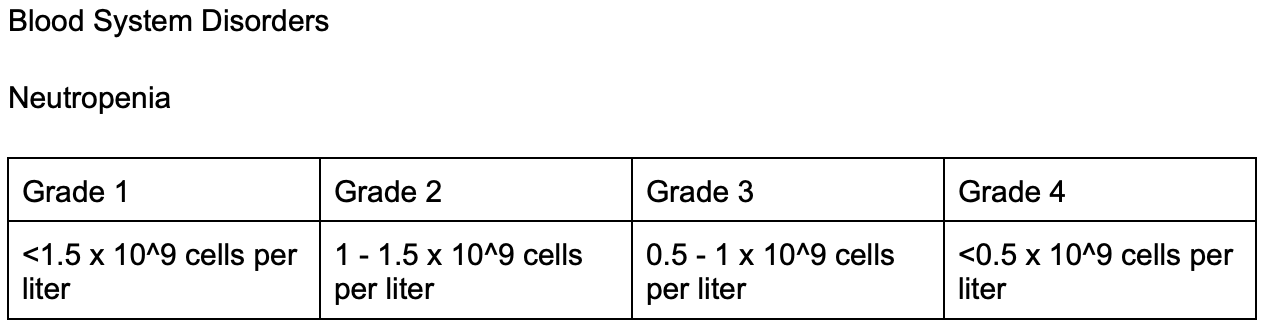
We don’t have time to go into febrile neutropenia today (good thing we’ve already covered it here), but we can touch on some thoughts to have with its related grading. Having less than 0.5 x10^9 neutrophils/L is usually alarming, so that makes the Grade 4+ score totally understandable. But what about a 2+ or 3+?
(Image)
Many times these types of labs can result in holding treatment and treating with filgrastim, a neutrophil stimulator. But it’s also useful to know ahead of time if a particular chemotherapy is prone to causing severe neutropenia (like doxorubicin or docetaxel). You can actually preemptively administer a neutrophil stimulator like pegfilgrastim to head off complications. Pretty much a pharmacy superpower, right?
Alright, now on to example #3.
Many chemotherapy drugs are renally cleared, so creatinine is a useful protein breakdown tool for evaluating renal function and dosing and adjusting medications. For example, carboplatin is dosed on AUC using the Calvert formula discussed here, but there are other drugs such as cisplatin and etoposide that are dosed based on creatinine clearance. So not only can we use the grading system to evaluate any damage a medication is doing to the kidneys, but we can also use the renal function lab results to manage future doses.
So as you can see, having these grading scales help provide a bit of a clue as to how we should manage adverse effects from our drug treatments. While labs and adverse effects may not tell the whole story, they are a huge resource, especially when you are starting out practicing and figuring out how to adjust dosing or continue treatment.
The tl;dr of the CTCAE Grading System
The Common Terminology Criteria for Adverse Events (CTCAE) Grading System is used to provide a standardized reference for adverse reactions with cancer drugs. It’s used in everything from research and development to clinical trials to clinical practice so that everyone is speaking apples to apples when evaluating. Here are the grades again to really lock them away in your minds!
Grade 1 - asymptomatic or mild symptoms, clinical observation only, no intervention needed
Grade 2 - mild to moderate symptoms, with maybe some local or noninvasive intervention
Grade 3 - severe and medically significant but not immediately life threatening
Grade 4 - life threatening consequences and urgent intervention is needed
Grade 5 - death