The Pharmacist's Guide to Sexually Transmitted Infections (STIs)
This article has been reviewed and is up-to-date as of 07/24/2024.
Brandon’s Note: Please join me in welcoming back Devki Gajera, PharmD, MPH. You may remember Devki from the (excellent and insightful) wisdom she previously gave us on Contraception and Race Coefficients in Clinical Equations. Devki received both her PharmD and MPH (Master’s in Public Health) from Virginia Commonwealth University prior to completing a PGY1 Community-Based Pharmacy Residency at the University of Virginia in Charlottesville. She currently works as an ambulatory clinic pharmacist. She does a great job merging her clinical skills with her public health perspective. You’ll learn a lot from this article, and I hope you enjoy it.
Take it away, Devki!
A Quick Intro to Sexually Transmitted Infections
The past year brought many changes in the infectious disease world (and not just due to the pandemic)! The Centers for Disease Control and Prevention (CDC) also updated their clinical guidelines for the management of sexually transmitted infections.
As the name implies, STIs are pathogens that cause infection through sexual contact.
I’m not an infectious disease expert. But I’ve worked in both community and ambulatory care pharmacy settings long enough to realize that STIs are prevalent enough to warrant an understanding of appropriate treatments. Just about every pharmacist would benefit from a better understanding of infectious diseases and how we treat them.
To get you started, here are a few articles on tl;dr that will help bring you up to speed:
Specifically speaking about STIs, the CDC has a handy pocket guide that is a lifesaver when you get asked questions by other providers. They also have a mobile app and wall chart if you want to get really fancy. You can hang the chart on your bedroom wall like an NSYNC poster circa 2000.
Sexually Transmitted Diseases vs Sexually Transmitted Infections
Right out of the gate, let’s clarify some terminology. You may have heard of Sexually Transmitted Diseases (STDs). That’s an older term that has fallen out of favor. We’ve switched over to Sexually Transmitted Infections (STIs). The reasoning for this switch is both to destigmatize the term STD and also be more specific as infections are not always chronic diseases.
An Overview of STI Guidelines
The STI guidelines are focused, most importantly, on preventing and controlling infections. There are five main goals:
Accurate risk assessment and education of people at risk (including changes in sexual behaviors and use of prevention services)
Pre-exposure vaccination for vaccine-preventable STIs
Identification of infected persons (both asymptomatic and symptomatic)
Diagnosis, treatment, counseling, and follow-up of persons infected with an STI
Evaluation, treatment, and counseling of sex partners of individuals infected with an STI
Recent Updates to the STI Guidelines
The 2021 STI guidelines are almost 200 pages (like most other guidelines!). In true tl;dr fashion, I’m here to hit the high points. I’m going to focus on the changes from the older 2015 version. And I’m going to really drill down on the areas most relevant to pharmacists. These include:
Treatment of Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis
Treatment of Pelvic Inflammatory Disease (PID)
Treatment of Mycoplasma genitalium
Treatment of Bacterial Vaginosis (BV)
Recommendations for Human Papillomavirus Vaccine (HPV) and counseling
Testing for Hepatitis C (HCV) infection
Testing for syphilis in pregnant women
How to Treat Niesseria gonorrhoeae, Chlamydia trachmoatis, and Trichomonas vaginalis
First, let’s get into one of the changes that will affect what medications are prescribed and dispensed: treatment of Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis.
For Neissseria gonorrhoeae, the guidelines recommend a single dose of ceftriaxone intramuscularly (IM).
Of note, this is a weight-based dose. For those weighing less than 150 kg, a 500 mg dose is appropriate. For all others, 1 g dose is recommended. Alternative regimens include:
gentamicin 240 mg IM once + azithromycin 2 g PO once
cefixime 800 mg PO once
What changed?
In the previous version of the STI guidelines, we used a combination of ceftriaxone and azithromycin, but at lower doses.
Specifically, we gave a single dose of ceftriaxone 250 mg IM + azithromycin 1g PO as single doses.
The dose increase recommended in the new guidelines is to target cultures with a higher minimum inhibitory concentration (MIC), especially in the context of azithromycin resistance in gonorrhea. Check out this scary-looking graph from the CDC:
Moving on…for Chlamydia trachomatis, the recommended regimen is doxycycline 100 mg PO twice daily for 7 days. Alternative regimens include:
azithromycin 1g PO for a single dose
levofloxacin 500 mg PO daily for 7 days
As a quick reminder, doxycycline was previously classified as Pregnancy Category D (remember when the FDA implemented the new Pregnancy and Lactation Labeling Rule (PLLR)? Yeah, me neither). Pregnant women should be treated with azithromycin first-line (especially in the second and third trimesters).
What changed?
Prior to this update, a single dose of azithromycin was recommended. However, a Cochrane review of 2,715 patients found higher rates of microbiologic treatment failure among men taking azithromycin vs. doxycycline (however, clinical failure was uncertain in both men and women). Observational studies have also found doxycycline to be more efficacious for rectal C. trachomatis than azithromycin.
If adherence is a concern, azithromycin as a single PO dose can be considered. But keep in mind, there are concerns for antibiotic resistance, specifically against azithromycin.
And lastly, for Trichomonas vaginalis, the recommendations are different for women and men.
Women: metronidazole 500 mg PO twice daily for 7 days
Men: metronidazole 2 g PO for a single dose
What changed?
Metronidazole 2 g PO for one dose is no longer recommended in women (note that it’s still the standard for men). A meta-analysis found that individuals who receive only one dose of metronidazole are 1.87 times more likely (95% CI: 1.23-2.82, p < 0.01) to fail treatment compared to those receiving multiple doses. Also of note, the vaginal gel formulation of metronidazole doesn’t reach therapeutic levels and is not recommended.
Me…reacting to the news that you actually CAN drink while on metronidazole
And here’s an earth-shattering point if you’re a few years out of pharmacy school…
There is no longer a need to counsel patients to avoid alcohol consumption while taking metronidazole!
Studies have shown no disulfiram-like interaction between ethanol and metronidazole. As someone who has counseled patients on avoiding alcohol with metronidazole countless times, this feels like a significant highlight.
So. What are the key takeaways for these three STIs? Antibiotic resistance continues to shift best practice. As with many infectious diseases, it’s a moving target.
For gonorrhea, we use ceftriaxone IM once. For chlamydia, we use doxycycline PO for 7 days (which is a longer duration than we previously used with single-dose azithromycin). Finally, for trichomoniasis, we use metronidazole PO for 7 days (which is also longer than our prior practice).
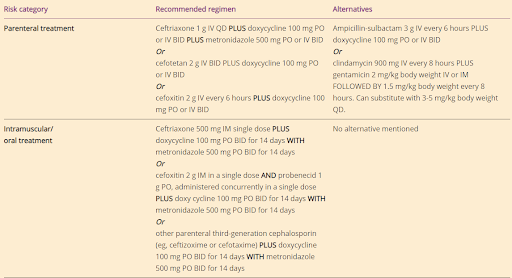
How to Treat Pelvic Inflammatory Disease (PID)
Let’s look at PID next. PID is an umbrella term for many inflammatory disorders that affect the upper female genital tract. It includes endometritis, tubo-ovarian abscess, and pelvic peritonitis. Almost 50% of women with PID have either N. gonorrhoeae or C. trachomatis. The treatment recommendations have changed slightly from 2015.
A combination of both IV and oral therapy for all PID cases is now recommended, and metronidazole is now added to all cases. The recommended regimen is:
ceftriaxone 500 mg IM once + doxycycline 100 mg PO twice daily for 14 days + metronidazole 500 mg PO twice daily for 14 days
The recommended alternative regimen is:
cefoxitin 2 g IM once + probenecid 1 g PO once + doxycycline 100 mg PO twice daily for 14 days + metronidazole 500 mg PO twice daily for 14 days
What changed?
Previously, metronidazole was only added if there was a tubo-ovarian abscess. However, a randomized controlled trial of 233 women found that women who received metronidazole vs not had less anaerobic organisms, less pelvic tenderness, and decreased Mycoplasma genitalium counts at 30 days after treatment. Metronidazole is now universally recommended since the risk of untreated anaerobes can include infertility, ectopic pregnancy, and tubal destruction.
Another change — Patients with intrauterine devices (IUDs) do NOT need to have the IUD removed unless there is a lack of clinical improvement within 72 hours of starting treatment. It was found that there is no clinical difference with IUD removal during treatment.
Of note, these studies have primarily included women with copper-containing and non-hormonal IUDs, so women with levonorgestrel-releasing IUDs should have a more detailed conversation with their providers.
What are the most important changes in PID management for you to remember?
Anaerobic coverage with metronidazole should routinely be part of the treatment regimen. The benefits outweigh the risks - it is a safe and effective add-on to the regimen. Just keep in mind that it contributes significantly to pill burden…and that could lead to non-adherence for some patients.
That’s a great opportunity for you to put on your pharmacist hat and discuss why it’s super important to complete the full course.
How to Treat Mycoplasma genitalium
Mycoplasma genitalium shows a causal association with PID, although individuals with PID don’t always have this organism and vice versa. Women with M. genitalium may have cervicitis (an inflamed cervix), preterm delivery, spontaneous abortions, and infertility. The complications in men are unclear and not well-studied.
The management of M. genitalium requires a two-part antibiotic course.
M. genitalium should be suspected in patients who have recurrent cervicitis and PID. Resistance testing isn’t always readily available, so we may have to work with empiric therapy.
It’s recommended to start with doxycycline 100 mg PO twice daily for 7 days followed by moxifloxacin 400 mg PO daily for 7 days. In this combination, the doxycycline works to reduce organism count and the moxifloxacin eradicates it.
That being said, if resistance testing IS available and the organism is found to be sensitive to macrolides, then azithromycin rather than moxifloxacin should be used following doxycycline. If you don’t have resistance testing, you should stick with moxifloxacin since the risk of macrolide resistance outweighs the benefit of using azithromycin over moxifloxacin.
As a final point, remember that M. genitalium is an atypical organism. It lacks a cell wall, therefore antibiotics that target cell-wall synthesis (including 𝛃-lactams) won’t work.
How to Treat Bacterial Vaginosis (BV)
Moving on to BV, which occurs when normal vaginal flora like Lactobacillus are replaced with anaerobic bacteria like G. vaginalis, Prevotella, or Mobiluncus to name a few. Most women with BV are asymptomatic, so it’s typical that only symptomatic women are treated.
What Changed?
The major guideline change for BV is the expansion of alternative treatment options. There are multiple choices for first-line treatment:
metronidazole 500 mg PO BID x 7 days
metronidazole gel intravaginally x 5 days
clindamycin cream intravaginally x 7 days
Alternative regimens now include clindamycin, secnidazole, and tinidazole. Of course, secnidazole is more expensive and has yet to show long-term effectiveness for BV compared with metronidazole.
In summary, BV can be managed with more than just run-of-the-mill metronidazole and clindamycin…but the other options may be more costly to patients. That said, there are copay assistance options available for secnidazole, metronidazole gel, and clindamycin vaginal cream.
Prevention of STIs
That wraps up updates in STI treatments, but that’s just half of the conversation around STIs. More than ever, infectious disease experts are also hyper-focused on prevention due to antibiotic resistance and the exceedingly obvious benefit to population and community health. Some highlights from the STI guideline updates include human papillomavirus (HPV) vaccination, syphilis testing in pregnant women, and hepatitis C virus (HCV) testing updates.
Human Papillomavirus (HPV) Vaccine Recommendations
Did you know that there are about 150 HPV types?! It flies under the radar because most people with HPV are asymptomatic and go unrecognized. Most individuals who get HPV usually clear the infection spontaneously, although it can cause genital warts, pre-cancers, and other cancers (cervical, anal, penile, vulvar, vaginal, head and neck).
HPV types 16 and 18 account for 66% of all cervical cancers, while types 6, 11, 31, 33, 45, 52, and 58 account for 15% of cervical cancers. For those keeping track at home, yes, that means that HPV is suspected to be the cause of more than 80% of all cervical cancers. Untreated HPV can also lead to anogenital warts. Luckily HPV vaccines have drastically reduced the incidence of both cancer (all types associated with HPV) and anogenital warts. Here are the relevant details:
There are three HPV vaccines licensed in the United States (Ceravrix, Gardasil, and Gardasil 9). The first two are 2-valent and 4-valent respectively, while the third is (you guessed it!) 9-valent. Any guesses as to which HPV types Gardasil 9 covers? Yep — 6, 11, 16, 18, 31, 33, 45, 52, and 58 (i.e. all the types we highlighted above!). For this reason, Gardasil 9 is the only vaccine that has been distributed in the U.S. since 2016.
Gardasil 9 is routinely recommended at 11 or 12 years of age, but can be given as early as 9 years of age. This may seem early, but we want to catch kids BEFORE they become sexually active, not after. Catch-up vaccination is available through 26 years of age. Benefit of vaccination diminishes after this, but patients 27 through 45 years of age who were not previously vaccinated may decide to get vaccinated through shared clinical decision-making with their provider. This includes males and females and is irrespective of sexual activity. The data for cancer prevention makes this an “everybody should get one” kind of recommendation. However, since this is an article focused on STIs, let’s be clear and spell out that there are also benefits in preventing anogenital warts for males and females. This includes men who have sex with men (MSM), transgender individuals, women who have sex with women (WSM), women who have sex with men and women, and immunocompromised males.
The STI guidelines now align with the Advisory Committee on Immunization Practices (ACIP) recommendation.
Hepatitis C Virus (HCV) Testing Recommendations
The World Health Organization (WHO) estimates that, globally, 50 million people have chronic HCV infection with about 1 million new infections occurring every year. Of those people, about 15-30% will develop cirrhosis in within 20 years. Unfortunately, there is no vaccine available, but treatment options have greatly expanded in the past 20 years. Check out this article we wrote a few years ago* or check out our antiviral cheat sheet for more info.
*Full disclosure, the HCV article is hella old (it’s sooooo 2016). We’re slowly working on updating our old articles, but until it is reaffirmed I would recommend referring to the current HCV treatment guidelines.
Universal HCV testing is recommended for anyone older than 18 years and with each pregnancy for women, routine HCV testing is recommended with individuals who have risk factors such as injectable drug use or hemodialysis.
The STI guidelines have adopted this recommendation, so they’re basically falling in line with what has been routine clinical practice for testing.
Though HCV is not efficiently transmitted through sex, it is shown to be increasingly prevalent among persons with HIV infection, MSM with HIV, and people with a history of high-risk sexual practices. Therefore, screening for HCV among individuals with specific sexual histories is recommended.
Syphilis Testing Recommendations
This is pretty straightforward. All pregnant women should be tested for syphilis at their first prenatal visit. Women who live in a community with high rates of syphilis or with other risk factors should be tested at the beginning of the third trimester. That part hasn’t changed. What has been updated with these STI guidelines is that the risk factors are more encompassing. They now include women with multiple sex partners, drug use or transactional sex, late entry to prenatal care, incarceration, and unstable housing. Ultimately, these are social determinants of health for poorer pregnancy-related health outcomes and so testing should be considered.
Summary of Changes to the STI Guidelines
The changes with HCV and syphilis testing as well as HPV vaccination aren’t exactly groundbreaking, are they? Many providers already are in line with this practice. However, these updates make the overall practice of pharmacy a little less muddy.
Now the ACIP and CDC are on the same page with HPV vaccines, syphilis testing is more inclusive for pregnant women, and testing for HCV now includes all adults regardless of risk factors.
Hopefully this information has been helpful to you. But, as a sort of tl;dr summary, here are the most important highlights of this article:
Gonorrhea is treated with a higher dose of ceftriaxone because of antibiotic resistance.
Chlamydia is treated with doxycycline for a longer duration because of antibiotic resistance.
Trichomonas is treated with metronidazole for a longer duration because of…YOU GOT IT…antibiotic resistance!
PID management should include metronidazole for anaerobic coverage.
Metronidazole with alcohol use is not clinically concerning and can be deleted from your medication counseling repertoire moving forward.
Antibiotic resistance continues to be navigated with stewardship strategies and a focus on preventive screenings and vaccines.