NICU Series Part 6: Neonatal Pharmacokinetics and Infectious Diseases
(Image)
Steph’s Note: Hey, pharmacy phriends! It’s been a minute. Okay, okay, more like nine months. (Hmmm… Was that an accident, or is it intentional that we waited 9 months for a post about babies? Hmmm…we’ll let you ruminate on that one… *wink).
After a bit of a hiatus (because, you know…life), we’re back with the next installment of our NICU miniseries! This time we’ll be talking about everyone’s favorite topic: infectious diseases (ID). Here to deliver this wealth of information is our pediatric and NICU guru, Dr. Courtney Howell. Teach us, Courtney!
Gretchen Wieners may not think ID belongs here, but we know better.
Welcome back, friends! Heads up - this one’s going to be a little different than the previous articles in the series.
Historically, we’ve primarily focused on premature neonates. But any baby, regardless of GA or size, is at risk for acquiring an infectious disease. Some more so than others, but we’ll get to that.
Don’t know what GA means? Take a peek at this article to brush up on some common NICU acronyms. Also make sure to check out the previous article in our series where we talked about vaccination considerations in the NICU setting.
Now, there are a lot of pathogens out there that cause a wide variety of diseases - too many to cover in a tl;dr article. So, we will be cherry-picking a select few that are likely to affect our neonatal population. We’ll also be talking about some pharmacokinetic considerations to help guide us in appropriately dosing and monitoring antimicrobial therapy in these patients.
Neonatal Pharmacokinetics
Neonatal pharmacokinetics are wildly different from those observed in adults. Hell, depending on the level of prematurity, they can even differ significantly from term infants.
So why in the world haven’t we covered this yet?
Well, if I’m being honest, we should have. This is really the first time I’ve ever tried my hand at writing, and when I started this series I was eager to introduce you to the specific disease states you may encounter in the NICU. And, since most of what we’ve covered so far has been related to those disease states and not very drug-heavy, I just didn’t think of it. I have failed you, and for that I am sorry.
Brandon, if you’re reading this, please don’t fire me.
BUT, I’m here to redeem myself! We’ll be covering lots of drugs in this article that are very much affected by pharmacokinetics, so let’s take a few minutes to get the lay of the land, shall we?
First things first, let’s define pharmacokinetics. In short, it’s what the body does to a drug. You probably remember this handy little acronym from school: ADME. That is:
Absorption
Distribution
Metabolism
Elimination
These are the processes the body uses to break down, use, and get rid of a drug.
As I said before, neonatal pharmacokinetics are very different from the adult ones you probably learned about. We’re going to highlight a few of the ways these processes can differ in neonates, but I think it’s important to note that we’re just barely scratching the surface here, and there’s a LOT more going on behind the scenes.
Absorption
Underdevelopment is one of the biggest factors contributing to the unique treatment of our neonatal patients. Take gastric absorption for example. Immaturity of, well, pretty much everything alters the way enteral medications are absorbed in neonates. Pancreatic and biliary systems, hepatic enzymes, GI flora…You get it. Prolonged gastric emptying time can also delay drug absorption.
One of the main drivers of gastric absorption in neonates is gastric pH. It’s a bit of a rollercoaster ride. It is neutral (pH ~6-8) at birth, then sharply declines to more acidic levels (~1-3) over the first 24-48 hours of life. After this, it returns to a neutral pH, then slowly becomes more acidic and reaches adult levels around two years of age.
If you’re like me and still get confused by which types of drugs are best absorbed in an acidic vs. basic environments, it might be time to review acid/base equilibrium and the Henderson-Hasselbach equation.
Basically, medications are better absorbed in their uncharged, non-ionized forms. To help keep this straight, check out the below equations for acid and base equilibrium in aqueous solutions:
As you can see in the red circles, acids are non-ionized when they are attached to a hydrogen ion (also called a proton), and bases are ionized when they are attached to a hydrogen ion (or “protonated”). Acidic solutions have more protons floating around, so the chances of one attaching to our acid or base are pretty good. So, in general, acids are better absorbed in acidic environments where they are often protonated (and uncharged), and bases are better absorbed in alkaline environments where they are NOT protonated (and uncharged).
I can’t be bothered with too much biochemistry or pharmaceutics, or my brain will simply shut down.
This is a ridiculous oversimplification (read here if you want more details about ionization and absorption), but it does help keep it straight.
So, if we consider the neutral/alkaline pH of a neonate’s stomach contents, it makes sense that weakly basic drugs like penicillin and erythromycin are better absorbed than weakly acidic drugs like phenobarbital and acetaminophen.
Other routes of administration are rarely used because absorption is so unpredictable.
You can kind of think of neonates like the little old lady that walks into your pharmacy. You know, the one that comes in for a vaccine and you just know you’re going to hit nothing but bone? Neonates also have low muscle mass (plus poor regional blood flow) making the intramuscular (IM) route less than ideal. There are exceptions, of course (shameless callback to the “eyes and thighs” regimen mentioned in our vaccine article!), but it’s not a route we utilize a whole lot in the neonatal population.
We also have to be careful with topical agents. A combination of thin skin and a high body surface area to mass ratio make neonatal skin ultra-permeable, and therefore more susceptible to systemic toxicity following topical administration. Prime examples include medications like corticosteroids and neomycin. Alcohols and other excipients are also a cause for concern, and not just in topical medications! Regardless of the route, preservative-free formulations should be used whenever possible in our neonatal population to avoid toxicity.
Rectal absorption in neonates is unpredictable due to variable GI motility and altered first-pass metabolism. Rectal contractions are also common and can expel medications from the site of absorption.
Distribution
I’d be willing to bet you didn’t learn a whole lot about neonatal medicine in pharmacy school. I’d also be willing to bet that one of the things you did learn about it is this: babies are little bags of water (or something to that effect).
Neonates have a relatively high total body water content. This proportion is even higher in preterm infants. A high total body water translates to a greater volume of distribution (Vd) for hydrophilic agents. Since these agents distribute to water (which, in a neonate is pretty much everywhere!), we generally need higher doses of these drugs to achieve desired peak concentrations.
A great example of this is gentamicin. For those of you in the adult world, think about the dose of gentamicin you’d give to a septic patient using traditional dosing. The dose may vary slightly depending on your peak goal, but probably somewhere between 1 and 2.5 mg/kg/dose, right? For the sake of simplicity, let’s say we’re giving our adult patient 1.5 mg/kg/dose to achieve a peak concentration of 6 mcg/mL.
Now let’s say we want to get that same peak of 6 mcg/mL in our neonatal patient. Remember, they’ll need more because of the high Vd for hydrophilic drugs. So maybe we’ll give them 2 mg/kg/dose? Or 3?
It’s actually going to be more like 4 or 5 mg/kg/dose!
So, to get the same result we got in our adult patient, we need to give our tiny little neonate about 3 times the dose. Crazy, right?!
Now, the dosing interval for these patients is NOT going to be the same. We’ll re-dose our adult patient every 8 (or maybe every 12) hours while our neonate won’t get another dose for 24 to 48 hours. The rationale for this will make more sense when we talk about excretion (the “E” in ADME), so stay tuned!
The main point here is that a smaller patient does NOT always mean less drug.
Protein binding can also affect the Vd. Neonates often have reduced concentrations of plasma proteins with decreased affinity for drugs which in turn can lead to an increased Vd for certain drugs like penicillin.
This alteration in protein binding can also cause problems when drugs like ceftriaxone and sulfonamides try to compete with bilirubin for albumin-binding sites. Neonates are prone to hyperbilirubinemia, and displacement of bilirubin from albumin can further increase levels of unconjugated bilirubin and increase the risk of kernicterus, a type of brain damage.
Metabolism
Drug metabolism and biotransformation can get really complicated, really quickly.
The tl;dr version is that most of these processes occur in the liver and, since neonates (especially those that are premature) have immature livers, they tend to metabolize drugs less well than their adult counterparts.
Expression of phase I and phase II enzymes vary with maturation and development. Most increase with age, but some are actually expressed at higher levels in fetal life and decrease after birth. The time to maturation varies depending on which source you’re looking at, but generally it’s around one year of life.
That’s not to say a one-year-old is walking (or crawling) around with an adult-sized, fully-functioning liver, but the CYP450 isoenzyme system is relatively developed.
Elimination
The last piece of pharmacokinetics, the “E” in ADME, is elimination. The liver contributes a little bit, but the kidney is the main workhorse here.
But this horse isn’t very good at its job. It’s overweight, sassy, and downright lazy.
The neonatal kidney is…how do I put this delicately?
(Image)
Let’s go back to our gentamicin example to illustrate this. If you recall, we landed on a typical traditional gentamicin dose of 1-2.5 mg/kg Q8-12H for an adult, and 4-5 mg/kg Q24-48H for our neonate.
But, wait…5 mg/kg Q24-48H? Doesn’t that sound like the extended interval dosing strategy we use in adults?
While they look similar on paper, they’re not the same. Since the gentamicin distributes all over the neonate’s body, we need a lot more drug to get the same effect. So it’s still traditional dosing (even if it doesn’t look like it).
But what’s going on with the dosing interval? If we’re saying a 1.5 mg/kg dose in an adult is essentially equivalent to a 4.5 mg/kg dose in a neonate (again, easy numbers for pretty math), why are we waiting so long to give the neonate their next dose? If we gave our adult patient their 1.5 mg/kg dose and then didn’t give them another one for say, 36 hours, that would do absolutely nothing for their infection. So why aren’t we re-dosing our neonate every 8-12 hours?
Remember, the neonatal kidney is stupid. She real dumb.
We have to give a whopping dose to get our desired peak concentration, but when it comes time to clear it, the kidney has no idea what to do.
This is especially true for premature neonates. The dose and dosing interval both need to increase with decreased PMA. Here’s a chart #StraightOuttaNeoFax® to help illustrate this:
That’s a wrap on our PK discussion. I want to reiterate that this was just an introduction. These are NOT the only things we need to consider when treating our neonatal patients, but it’s a good place to start.
Now let’s get to the good stuff: ID.
*Quick disclaimer: I’m going to be using a lot of gender-specific terms like “Mom”, “pregnant woman”, and “breastfeeding”. It is always important to assess and use your patient’s preferred terminology when counseling.*
Neonatal Sepsis
We’ll start with neonatal sepsis. There are two distinct types classified by time: early-onset sepsis (EOS) and late-onset sepsis (LOS). We’ll talk about the differences in a minute, but first let’s take a look at their similarities.
Infants presenting with neonatal sepsis display symptoms we typically think of when we hear “sepsis” (e.g., temperature instability, heart rate instability, cyanosis/pallor, respiratory distress, hypotension, etc.). They also display symptoms more specific to neonates such as bulging fontanelles (the super scary soft spots on babies’ heads) and feeding intolerance. It’s important to note that the definition of EOS and LOS is microbiologic rather than functional like it is in adults and older pediatric patients.
Diagnostic and laboratory tests vary based on the institution and clinical presentation of your patient, but they probably include some combination of blood cultures, cerebral spinal fluid (CSF) cultures, and maybe a urine culture. Other labs may include a complete blood cell count (CBC) and inflammatory markers like C-reactive protein (CRP). However, only blood and CSF cultures can be used to diagnose EOS. The other tests give us a little more information, but are not diagnostic.
Now, let’s tease apart EOS and LOS.
Timing and Risk Factors
EOS presents within 72 hours of birth, while LOS presents > 72 hours after birth. This makes sense when you think about the source.
If you’ve ever had the pleasure(?) of getting “the swab”, you know what I’m talking about.
Bacteria resulting in EOS comes from Mom. Transmission can occur in utero through the placenta or can be horizontal (i.e., through physical contact during labor and delivery). Certain factors such as prematurity*, prolonged rupture of amniotic membranes, maternal chorioamnionitis, and maternal Group B Streptococcus (GBS) colonization can increase the risk of EOS.
Speaking of GBS, it’s the most common pathogen in EOS. E. coli comes in second, followed by some other gram-positive and gram-negative organisms. The good news? Routine testing for GBS is done between 36 and 38 weeks of pregnancy. GBS is present in about 1 in 4 pregnant women, and the bacteria can come and go (hence why we test so close to delivery). It is NOT a sexually transmitted infection (STI). Intrapartum antibiotics (usually penicillin or ampicillin in the absence of allergies) can be administered during labor to prevent bacterial transmission.
*Side note - The epidemiology of EOS differs significantly between term/late-preterm infants and very preterm infants. So much so that the American Academy of Pediatrics (AAP) actually has separate guidelines for neonates born at < 35 weeks’ gestation and those born at ≥ 35 weeks’ gestation with suspected or proven EOS. The nuances are outside the scope of this article. I’ll mainly be citing the guideline for those ≥ 35 weeks’ gestation since, unless you’re working in a pediatric hospital, you probably aren’t taking care of very preterm infants.
On the other hand, LOS comes from the environment. Inpatient interventions like central venous catheterization and intubation can introduce bacteria, leading to infection. It can also result from contact with healthcare workers and caregivers.
For LOS, coagulase-negative staphylococcus (CoNS) and S. aureus top the list of common pathogens.
Empiric Management of Neonatal Sepsis
Empiric antibiotics should be started in infants with early-onset risk factors (described previously) or in those with suspected infection. The mainstay empirical therapy for EOS is ampicillin + gentamicin*. This regimen covers all the main pathogens (i.e., the “Mom bugs”). Although resistance rates are increasing, the majority of pathogens are still susceptible to this combination. Extended-spectrum antibiotics are not typically recommended, except in the case of critical illness.
*WARNING: Going on a little side tangent here. Guidelines previously recommended cefotaxime as an alternative to gentamicin for empiric treatment of EOS. However, Hospira stopped making cefotaxime and it was added to the Orange Book’s Discontinued Drug Product List back in 2018. The FDA did allow for temporary importation to address the shortage, but institutions pivoted away from the drug and ceftazidime became the new go-to third generation cephalosporin.
Another thing you probably heard in school is that neonates can NOT have ceftriaxone. This is partially true, but there are (almost) always exceptions. Specific criteria likely vary by institution, but UCSF’s Infectious Disease Management Program has a pretty good set of safety criteria. Here are the biggest considerations:
Level of prematurity
USCF’s guideline says candidates must have a PNA ≥14 days and CGA ≥41 weeks.
Bilirubin levels
Recall our discussion about ceftriaxone and bilirubin displacement in the PK section at the beginning of this article!
Receipt of IV calcium-containing solutions
Crystalline ceftriaxone-calcium deposits in the vasculature resulting in death and other serious adverse events has been reported in neonates. Calcium-containing IV products should not be given to neonates within 48 hours of ceftriaxone (regardless of whether it was given IV or IM). Unfortunately, many infants in the NICU require calcium-containing total parenteral nutrition (TPN), so this rules out much of the population.
Some of these criteria may be bypassed if giving a single dose of ceftriaxone for ophthalmia neonatorum (ON) (yet another shameless callback to the “eyes and thighs” regimen mentioned in our vaccine article!). We’ll talk about this condition more in a bit.
So anyway, the moral of the story is that if you see ceftriaxone in a neonate you should be wary, but it’s not necessarily wrong.
Okay, tangent over. Back to our regularly scheduled programming.
If blood cultures (which hopefully were drawn BEFORE antibiotics were started) are sterile at 36 to 48 hours, empiric therapy may be discontinued. Therapeutic aminoglycoside monitoring is not necessarily warranted if only used during this rule-out period. If EOS is confirmed by blood culture, a lumbar puncture should be done to rule out meningitis. Definitive therapy and duration should be guided by pathogens and the results of serial blood cultures/cerebrospinal fluid (CSF) analysis.
Continuation of therapy may be justified in infants with unexplained cardiorespiratory illness, but is not generally recommended in well-appearing infants with laboratory abnormalities alone. If therapy is continued, therapeutic aminoglycoside monitoring is recommended. Trough goals are usually < 1-2 mg/L.
Moving on to LOS! The standard empiric regimen here is vancomycin + gentamicin.
Blood cultures are recommended here, too. If there is a clear source (like a central line), cultures should also be taken from this site. CSF analysis should be done as meningitis is more commonly a late-onset infection. Urine cultures have more clinical utility after the first three days of life, so these may be helpful as well.
I honestly kind of forgot other formulations of amphotericin B exist. It’s always “AmBisome this, AmBisome that”.
Candida species may also be the culprit of late-onset infections. They can be treated with amphotericin B or fluconazole. Fun fact: the conventional amphotericin B formulation (deoxycholate) is actually preferred over the lipid formulations in neonates due to better penetration into the CNS, urinary tract, and eyes -- all common infection sites in the neonatal population. Neonates also tend to tolerate this formulation pretty well compared to adults.
I feel like it was really drilled into our heads in school that the liposomal formulation is preferred, but here’s a situation where that’s not necessarily the case! Also a good opportunity to remind you to ALWAYS double-check (and, in the case of a neonate, TRIPLE-check) your amphotericin B dosing. With all the formulations and variable dosing, that drug is just a med error waiting to happen.
Infants at institutions with high prevalence (> 10%) of invasive candidiasis may benefit from antifungal prophylaxis, but it’s not routine practice.
Ophthalmia Neonatorum (ON)
Told you we’d come back to ON! I already put in two shameless plugs for our vaccine article (if I do it again will you actually read it?), but now I’m going to put one in for our (recently updated!) STI article.
Why, you ask? Because the same guidelines we cited in that article also cover this condition!
The two big bad bugs we’re worried about here are Chlamydia trachomatis and Neisseria gonorrhoeae. If you read the vaccine article (cough cough), you’d know that erythromycin ophthalmic ointment is routinely given shortly after birth to prevent gonococcal ON (and non-gonococcal neonatal conjunctivitis caused by other bacteria).
Unfortunately, this prophylactic regimen is ineffective against C. trachomatis. Appropriate prenatal care and routine C. trachomatis screening is crucial to prevent these infections. If an infection does occur, systemic therapy is required. The recommended treatment is erythromycin 50 mg/kg/day divided into four doses for 14 days. The efficacy of this regimen is only about 80%, so a second course may be required.
There is some data for azithromycin as well, and it may be more tolerable (we actually sometimes use erythromycin to promote intestinal motility in the context of feeding intolerance, so think lots of diarrhea!). It’s important to note that both of these agents have been associated with infantile hypertrophic pyloric stenosis (IHPS) among infants less than 6 weeks of age, so infants receiving either of these drugs should be monitored for symptoms.
ON caused by N. gonorrhoeae IS preventable by administration of erythromycin ophthalmic ointment, but prenatal screening is just as important. Ointment should be applied as soon as possible after birth regardless of the method of delivery (vaginal or cesarean). If an infection does occur, a single dose of ceftriaxone 25-50 mg/kg IV or IM (MAX 250 mg) is recommended. Recall our previous discussion on ceftriaxone in the “Neonatal Sepsis” section above. The guidelines recommend cefotaxime as an alternative for those unable to receive ceftriaxone but, again, we don’t make that any more…I’d say this would be a great time to exercise your interprofessional collaboration skills and consult ID!
TORCH Infections
The last piece of our ID discussion will be TORCH. (No, not like the one used to light the olympic cauldron…But how good were the games this year?!)
TORCH is actually an acronym for a group of infections that can cause congenital conditions or abnormalities if a baby is exposed to them in utero. It stands for: Toxoplasmosis, Other, Rubella, Cytomegalovirus (CMV), and Herpes Simplex Virus (HSV).
Toxoplasmosis
Toxoplasmosis is a disease caused by Toxoplasma gondii, a parasite found in raw/undercooked meat and eggs, soil, and cat feces. It usually spreads by oral contact. Pregnant women should take precautions, such as avoiding consumption of undercooked meat and eggs (RIP raw cookie dough), avoiding yard work that involves direct contact with soil, and wearing gloves when changing cat litter to prevent infection. The risk of maternal transmission is actually pretty low in the first trimester but increases as the pregnancy progresses. Pregnant women aren’t routinely screened for this condition in the U.S., so those that think they may have been exposed should talk to their provider.
Most babies born with congenital Toxoplasmosis (CT) have no symptoms. As the baby ages, some symptoms may include swollen lymph nodes, easy bruising, jaundice, anemia, and an enlarged liver or spleen. If left untreated, severe complications can occur. These include chorioretinitis (inflammation of the retina that can lead to blindness), hydrocephalus (a build-up of CSF in the brain), and intracranial calcifications, which can lead to intellectual disability, developmental delays, and hearing loss.
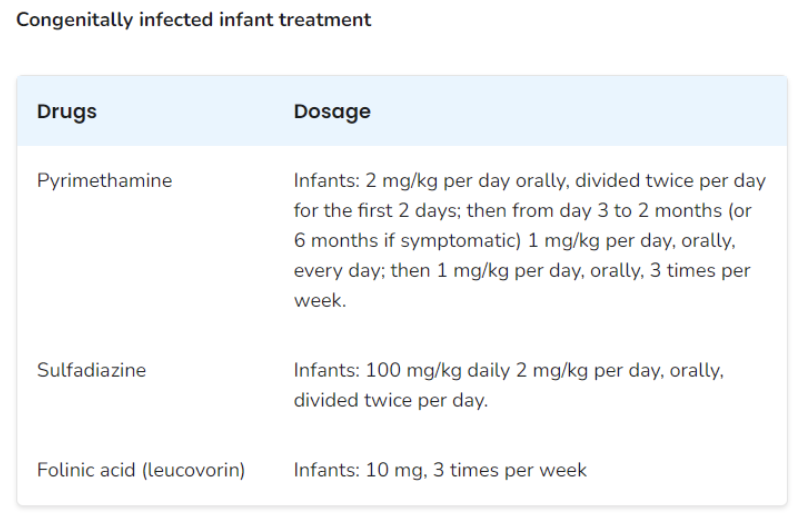
According to the U.S. Centers for Disease Control and Prevention (CDC), pregnant women can be treated with spiramycin if the infection was acquired before 18 weeks gestation and is not documented or suspected in the fetus. Spiramycin is not commercially available in the U.S., but it can be obtained from the U.S. Food and Drug Administration (FDA) for this indication. A combination of pyrimethamine, sulfadiazine, and leucovorin is recommended for infections acquired after 18 weeks gestation or if it is documented or suspected in the fetus.
Congenitally infected newborns are also treated with a combination of pyrimethamine, sulfadiazine, and leucovorin for at least 12 months. The dosing recommendations can be found in the table below:
Other
I know, I know…“other”. Super helpful, right? Well, unless you want the acronym to be TSVPHRCH, you’re gonna have to get over it.
“Other” encompasses a variety of viral and bacterial infections, including syphilis, varicella, parvovirus, and human immunodeficiency virus (HIV). Since a majority of these infections are well-known, I’ll leave out a lot of the background and just hit the high points.
According to the 2021 CDC STI guidelines, all pregnant women should be tested for syphilis at their first prenatal visit (even if they’ve been tested previously). About half of babies infected with syphilis before birth don’t make it to term or die shortly after. Untreated congenital syphilis can lead to severe complications, including neurological and cardiovascular problems, blindness, and deafness.
Pregnant women infected with syphilis should be treated with IM penicillin G to prevent transmission to the fetus. Note that this is the ONLY treatment shown to be effective in treating fetal infection and preventing congenital syphilis, so pregnant women with penicillin allergies should be desensitized and treated in a hospital. Dosing and duration will depend on the stage of infection.
Penicillin G is also the recommended treatment for infants with congenital syphilis. Dosing, route, and duration will depend on the likelihood of infection. Note that there are multiple formulations of penicillin G, and the ONLY one that can be given IV is the aqueous crystalline formulation. The others (benzathine and procaine) must be administered IM.
At the time of this writing, we are experiencing a penicillin G benzathine (Bicillin-LA) shortage due to a surge in syphilis cases. Refer to your institution for guidance regarding conservation.
Varicella is a disease caused by the varicella-zoster virus. Babies born with congenital varicella syndrome may experience a variety of birth defects including limb, brain, eye, and autonomic nervous system abnormalities, scarring on the skin, and mental retardation. The good news? There’s a vaccine for that! However, it is a live vaccine which means it’s contraindicated in pregnancy, so ideally individuals would be vaccinated before getting pregnant. Those who have received the vaccine (or have immunity from a prior infection) are very unlikely to transmit an infection to their baby.
For those unable to receive the vaccine or those that have been exposed to the virus, varicella-zoster immune globulin (VZIG) is available to prevent or reduce the severity of infection. Newborns may also receive VZIG to prevent or treat varicella if they meet certain criteria. Acyclovir therapy may also be recommended for infected pregnant women and is the treatment of choice for newborns with congenital varicella.
Congenital fifth disease, caused by parvovirus B19, is a viral illness that is usually mild in adults but can lead to fetal complications. About half of adults have already been exposed and are immune to this illness. Transmission only occurs in about 5% of cases but can be severe when it does. The virus affects the baby’s ability to produce red blood cells which can lead to severe anemia, heart failure, and death. There are currently no medications available to treat parvovirus B19, so providers will closely monitor fetal development and may recommend intrauterine blood transfusions for affected mothers.
HIV is a disease that’s generally covered pretty extensively in pharmacy school. In case you need a general HIV refresher, here’s an incredible article written by one of our (even more incredible) team members! In this article, we’re going to focus on pregnant women and prevention of vertical disease transmission. You could read the entire (637 page!) perinatal HIV guidelines, but I’m guessing you’re looking for some lighter reading. So, again, let’s hit the high points.
HIV is also included in routine prenatal screening. Like syphilis, the CDC recommends all pregnant women be tested at their first prenatal visit, even if they’ve been tested previously. Antiretroviral therapy (ART) is safe and effective in pregnancy and should be initiated as soon as possible. ART regimens should be individualized for each pregnant woman. For those in labor with unknown HIV status or unknown viral load, intrapartum zidovudine is recommended to prevent vertical transmission (think intrapartum antibiotics to prevent GBS transmission during labor!). Scheduled cesarean delivery may be recommended for certain pregnant patients with HIV who have not achieved viral suppression on ART.
In terms of infant feeding, breastfeeding is not routinely recommended because the virus can be transmitted through breast milk. The risk of transmission decreases to less than 1% if the mother has achieved viral suppression with ART, but the risk is still there. It’s important for those with HIV to have a discussion with their provider to make the best choice for feeding their infant.
Rubella
*Sips tea*
All jokes aside— While I am very much pro-vaccination, I also respect the fact that everyone has the right to refuse any medical treatment for themselves or their children. I encourage anyone who has concerns about vaccines to talk with a trusted healthcare provider to help make the best decision for your family.
Rubella (AKA “German measles”) is caused by a virus called rubivirus. The virus usually causes mild disease in infants and children, but can be incredibly severe for a baby when contracted in utero, especially in the first trimester. Pretty much every organ system can be affected. Congenital rubella syndrome (CRS) can lead to heart problems, eye problems, deafness, intellectual and learning disabilities, developmental delays, diabetes, and bleeding problems. Unfortunately, there is no cure for CRS. Symptoms may be able to be managed depending on the severity, but infection prevention is crucial. Pregnant women who have been exposed to rubella should seek medical attention immediately.
But, again, I have good news. We also have a vaccine for rubella, and it is all but eradicated in the United States. It is part of the measles, mumps, and rubella (MMR) vaccine. This is also a live vaccine so, like the varicella vaccine, it is also contraindicated in pregnancy.
CMV
CMV, a member of the herpesvirus family, is an incredibly common infection. In fact, it’s estimated that half of adults are or have been infected by the time they turn thirty. It may present similarly to mononucleosis, but it generally causes no symptoms in healthy adults. However, complications can occur when a woman becomes infected for the first time during pregnancy.
Most babies won’t have symptoms of congenital CMV at birth, but these may include premature birth, low birth weight, liver/spleen enlargement, jaundice, anemia, rash, and seizures. Serious complications can occur, including hearing loss, visual impairment, mental retardation, autism, epilepsy, and death.
Antiviral treatment with IV ganciclovir (6 mg/kg Q12H) or PO valganciclovir (16 mg/kg Q12H) may improve hearing and neurodevelopmental outcomes in infants with congenital CMV, especially if initiated within the first month of life. Valganciclovir is just the prodrug of ganciclovir, so their side effect profiles are similar. Neutropenia, thrombocytopenia, renal toxicity, and hepatotoxicity are all possible, and labs should be monitored accordingly.
Ganciclovir seems to have a slightly higher rate of toxicity. It also carries the risk of extravasation, so oral valganciclovir therapy is usually preferred and should be utilized as soon as possible. Patients should be treated with antivirals for a minimum of 6 weeks but courses could be up to 6 months depending on the clinical situation. Foscarnet and cidofovir may be considered in the setting of ganciclovir resistance or severe toxicity, but routine use is not recommended.
HSV
Last but not least, we have HSV. The virus can be transmitted from mother to baby before (less common), during (more common), or after (also less common) birth. Some studies have shown that cesarean delivery can decrease the risk of transmission. Routine prenatal screening of HSV-1 and HSV-2 is not currently recommended, but those at high risk of acquiring HSV (especially in the third trimester) should be counseled appropriately. For those with known recurrent genital herpes, suppression therapy with acyclovir or valacyclovir is recommended starting at 36 weeks gestation.
Symptoms at birth may include irritability, blisters, trouble breathing, jaundice, and easy bruising/bleeding. Infections can manifest in three ways:
Localized lesions on the skin, eyes, and mouth
CNS disease (encephalitis), and
Disseminated infection.
Disseminated infection is the most dangerous as it can affect multiple organs, including the liver, brain, lungs, and kidneys. If left untreated, the latter two manifestations can be fatal.
The mainstay of treatment for neonatal HSV infection is IV acyclovir at a dose of 20 mg/kg Q8H. The interval may be extended to Q12H for infants born at less than 34 weeks PMA per NeoFax®. A duration of 14 days is appropriate when disease is limited to the skin and mucous membranes, but should be extended to 21 days for disseminated disease. Adequate hydration is recommended to prevent renal toxicity, and absolute neutrophil count (ANC) should be monitored due to risk of neutropenia.
The tl;dr of Neonatal PK and Infectious Diseases
And there you have it! That was a LOT of information, so here’s a little summary:
Neonates are not just smaller versions of adults. In fact, they’re not even just smaller versions of kids! Pharmacokinetic processes differ greatly between these populations, and medications should be dosed and administered accordingly.
Albeit similar, there are major differences between early- and late-onset sepsis. EOS pathogens generally come from Mom, while LOS pathogens come from the environment.
Other pathogens from Mom (thanks, Mom…) can cause ON and TORCH infections, all of which can lead to congenital conditions and abnormalities. Some are treatable while others, unfortunately, are not.
Join us next time for the LAST installment of this series where we’ll talk about fluids and nutrition. I’ll try to get it to you just a liiiiittle sooner!