NICU Series Part 2: Patent Ductus Arteriosus (PDA) in Premature Neonates
Steph’s Note: This week, we’re continuing our newfound NICU momentum with the second chapter of our neonatal pharmacology knowledge drop. We have Courtney Howell, PharmD, to thank for all this phenomenal info! If you haven’t noticed from her first post on respiratory issues in premature neonates, she’s pretty amazing at taking complex concepts and breaking them down into more palatable tidbits. This post is no exception, so take it away, Courtney!
And BTW…we’ve got a super handy Pediatric Pharmacy Pocket Guide that gives you the ins and outs of all things peds. It’s an absolute life-saver…especially if you only occasionally come across pediatric patients in your practice. Check it out here!
(Image)
Welcome back, friends! In this installment of our Neonatal Intensive Care Unit (NICU) miniseries, we will be talking about the most common complication of the cardiovascular system in premature infants. As I mentioned in the first NICU miniseries post on respiratory issues, this is not all-inclusive of every cardiovascular complication you may encounter in the NICU.
There is a reason this is called tl;dr pharmacy. If we make these articles too long, no one is going to read them! It’s just science.
FYI, I’ll be using some of the age and weight-related abbreviations from the previous article, so make sure to take a peek at that before you read on. Without further ado, let’s get started!
What is PDA?
Seriously, guys. No one wants to see that. Don’t be nasty. (Image)
Ahhhh, love. When two people show affection for one another in a public setting… Oh, wait! Wrong kind of PDA.
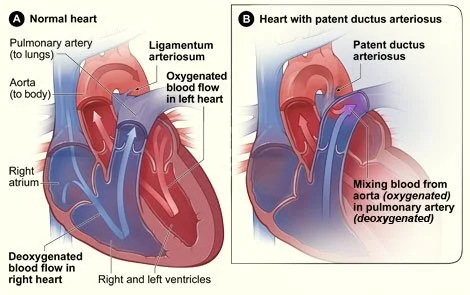
The PDA we’re talking about is patent ductus arteriosus. This is a congenital heart defect that occurs more frequently in preterm infants. During gestation, the two major arteries leaving the heart (the aorta and pulmonary artery) are connected. The bridge between the two is called the ductus arteriosus (DA).
The placenta serves as the primary source of oxygenation during development, so the DA diverts the baby’s blood away from the lungs and shunts it to the placenta. After birth, pulmonary pressures and vascular resistance are altered which make the lungs the source of oxygenation and gas exchange and render the DA unnecessary.
(Image)
A number of mechanisms are involved in keeping the DA open (i.e., maintaining patency) and closing it upon birth. In normal term infants, the DA closes within 96 hours. Preterm infants have immature structural components involved in ductal closure, so the earlier a baby is born, the longer it takes for the DA to close, and the higher the risk it will remain open.
Several hemodynamic, environmental, and genetic factors may also increase the risk of PDA. When the DA remains open, a continuous left-to-right shunt develops. This can result in increased pulmonary blood flow which leads to increased pulmonary arterial pressure, edema, reduced lung compliance, and impaired oxygenation. Premature neonates are especially susceptible to these effects as a result of organ immaturity.
My patient has PDA. Now what?
Not all PDAs are clinically significant, and not all require treatment. If the remaining hole is small, there may not be enough of a difference in pressures to cause problems. Some people don’t find out they have a PDA until childhood or even adulthood.
The criteria for PDA closure are not clearly defined. Determining whether a PDA is hemodynamically significant (hsPDA) should be based on a combination of echocardiographic and clinical variables, including individual risk factors for adverse outcome (e.g., GA, PNA, and comorbidities).
I’m sorry, what were we talking about again?
Observational studies suggest babies born with a hsPDA may be at a higher risk for bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (IVH), and necrotizing enterocolitis (NEC). If you’ll recall, the left-to-right shunt caused by a PDA can lead to breathing problems and edema (at least I hope you remember, we literally talked about it like 30 seconds ago). This can increase the need for mechanical ventilation, increasing the risk for lung injury and BPD (aka chronic lung disease (CLD)). The shunt may also cause fluctuations in cerebral blood flow, increasing the risk for IVH, as well as decreased intestinal blood flow, increasing the risk for NEC. Interestingly, closure of the DA has not been shown to decrease the rate of any of these comorbidities, so further studies are needed to establish their relationship and causality.
hsPDA Prophylaxis and Treatment
Because you took the time to click on and read the link I provided about the mechanisms involved in keeping the DA open (I know you didn’t, but I’ll let it slide), you know that prostaglandin E (PGE) is one of the dilating factors involved in the patency pathway. After birth, PGE levels fall which, along with many other factors, leads to closure of the DA.
Because of this involvement of prostaglandins, the cyclooxygenase (COX) inhibitors indomethacin and ibuprofen, which inhibit prostaglandin synthesis, are the agents of choice for pharmacological prevention and treatment for ductal closure. I know what you’re thinking, “Did you just suggest using ibuprofen on an infant less than 6 months of age?! I thought you weren’t supposed to do that!”
While this is true for pain and fever dosing, there is an indication for these medications for PDA closure in neonates. (In fact, there are a lot of exceptions to the “rules” in neonatal and pediatric medicine, we just don’t learn a lot of them in school. Or maybe they are taught, but they’re probably the subject of one exam or are glossed over once and then never talked about again.)
How I feel when I try to remember anything from pharmacy school. (Image)
Again, I want you to think wayyyyy back to your pharmacology class and find the file in your brain labeled “COX inhibitors.” If you recall, the same mechanism that makes them useful also makes them toxic. Prostaglandins are involved in the homeostasis and protection of many organs, so inhibition of COX (and therefore prostaglandin synthesis) can lead to reduced blood flow and damage to the kidneys, gastrointestinal (GI) tract, central nervous system (CNS), etc.
For years, indomethacin was the drug of choice for PDA prevention and closure, but ibuprofen is emerging as the preferred treatment agent due to its similar efficacy and improved safety profile (namely decreased renal and GI adverse events) when compared to indomethacin. However, indomethacin is still the preferred agent for PDA prophylaxis as it was found to decrease the incidence of IVH in the Trial of Indomethacin Prophylaxis in Preterm Infants (TIPP).
When used prophylactically, indomethacin is usually dosed at 0.1 to 0.2 mg/kg IV every 12 to 24 hours beginning within the first 6 to 24 hours after birth for a total of 3 doses. A retrospective study found that the incidence of IVH was not decreased with administration of indomethacin prior to 6 hours of life. The optimal indomethacin dose is unknown (I mean, that’s a pretty washy range I just gave you), but 0.1 mg/kg IV every 24 hours for 3 doses may reduce the risk of oliguria (i.e., decreased urine output (UOP)).
Wanna hear a fun fact about neonates?
Even though their muscle mass is extremely low, serum creatinine (SCr) levels can mimic that of an adult for the first week or so due to transfer of some of the mother’s creatinine from the placenta. Immature renal function of premature neonates can also muddy the waters, so UOP can sometimes be a better measure of kidney function in the NICU. The sweet spot is about 1-3 mL/kg/hr. If UOP drops too low (< 0.6 mL/kg/hr), hold subsequent doses of NSAIDs and consider holding or increasing the interval of other nephrotoxic medications.
Also, just because a low UOP is bad does not mean a high UOP is good. Since the kidneys are still maturing, sometimes a high UOP (usually 5 mL/kg/hr and up) can signal high-output renal failure.
Treatment doses of indomethacin are usually given at 12- to 24-hour intervals in a maximum of two courses with three IV doses per course. That being said, longer treatment courses (0.2 mg/kg every 24 hours for a total of 5 to 7 days) have been used. Treatment doses vary by PNA and are summarized in the table at the end of this article.
Standard ibuprofen dosing for PDA closure is 10 mg/kg PO or IV, followed by 5 mg/kg at 24 and 48 hours. A second course may be used if needed. High-dose therapy (15 to 20 mg/kg PO followed by 7.5 to 10 mg/kg PO daily for a total of 3 doses) may increase the success rate of PDA closure, but it has not been found to improve other outcomes. IV ibuprofen is not recommended in the first 24 hours of life due to increased risks for renal failure, GI hemorrhage, and persistent pulmonary hypertension of the newborn (PPHN).
Acetaminophen may also be used to close a PDA. It is dosed at 15 mg/kg PO every 6 hours for 3 days with the option to repeat the course if needed. An analysis of the Patent Ductus Arteriosus: TO LEave It alone or Respond and Treat Early (PDA-TOLERATE) trial found closure rates to be much lower with acetaminophen than indomethacin or ibuprofen among infants < 28 weeks GA. Other trials that enrolled infants with a higher GA found similar rates of closure between the three treatment options, so acetaminophen may be beneficial, especially if COX inhibitors are contraindicated or ineffective.
How do we decide when to treat?
(Image)
This is a complex question with a complex answer. There are many different factors (genetics, altitude, fluid and respiratory management) that can affect the rate of spontaneous PDA closure in a given NICU. Spontaneous closure is common in general, so in many cases pharmacological and surgical measures are falling out of favor. “Watchful waiting” and/or conservative treatment with fluid restriction, diuretics, and targeted ventilation is becoming more common.
In fact, surgical ligation has been linked to poor neurodevelopmental outcomes and other comorbidities and should be reserved for rescue therapy when pharmacological therapy fails. These interventions can still be useful in certain patients.
Prophylaxis with indomethacin may be beneficial in patients < 26W GA and weighing < 750 g in NICUs with a low rate of spontaneous closure. Results from the PDA-TOLERATE trial suggest early pharmacological treatment (< 6 days after birth) with ibuprofen should be considered for infants < 28W GA with a hsPDA and greater than minimal respiratory support. Symptomatic treatment (≥ 6 days after birth) should be considered for VLBW infants with a hsPDA and greater than minimal respiratory support. If late or rescue therapy is warranted, a combination of pharmacological and surgical interventions may be used.
There are no RCTs evaluating pharmacologic closure vs. catheter-based closure vs. surgical ligation, but the logical step-wise approach would be treatment with a COX inhibitor, a trial of acetaminophen (although success rate is likely to be low), then surgical intervention if deemed necessary by the neonatologist. These recommendations, derived from a state-of-the-art review article published by the American Academy of Pediatrics (AAP), are mostly based on expert opinion and must be adapted and modified at the local practice level.
The tl;dr of PDA
How are we doing? Is everything clear as mud?
PDAs are complex. They can be no problem at all, or they can be a really big problem (especially in our littlest of littles!). The decision whether to treat the PDA should be based on a combination of echocardiographic and clinical variables.
In recent years, treatment has shifted towards a more conservative approach. Historically, indomethacin has been the preferred drug for pharmacological prevention and closure of PDA, but ibuprofen is emerging as the preferred treatment agent thanks to its improved safety profile (although indomethacin is still the drug of choice for prophylaxis due to a decreased risk of IVH associated with its use). Acetaminophen may be a good option if NSAID therapy is contraindicated or ineffective, but data regarding its efficacy is mixed. Surgical intervention should be reserved for rescue therapy in select patients.
Here’s a nice li’l dosing summary table for ya so you don’t need to look back through the article.