New Drug Approval: Suzetrigine (Journavx)
(Image)
Steph’s Note: This week, we’re bringing back an old friend that we haven’t seen in quite a while - the new drug approval post. It’s not that we don’t like these posts or that there haven’t been worthy candidates to write about, but really, this one was just too interesting NOT to write about. First, I mean, who puts a v next to an x in a name, and how the heck are we supposed to pronounce it? So many questions. Let’s get some answers…to this and other (much more) important questions, I promise.
For the past 10+ years, the opioid crisis has been the skeleton finally exposed in the pharmacy world’s closet. A constant in news feeds (for good reason), it’s been a slew of shocking numbers, horrific and tragic stories, and finger pointing. Looots of finger pointing.
Then came the stories about law suits, the changes to prescribing rules, and the enhancements for monitoring patients and prescribers. All due to this opioid class of medications with its addictive and potentially life-threatening properties.
In response, we’ve seen upticks in attempts to utilize alternative classes of medications to treat pain syndromes. Who here doesn’t dispense gabapentin (or type it on a medication history list) at least 5 gazillion times a day? What about meloxicam or ibuprofen? We’ve been reaching for new uses for acetaminophen as an opioid-sparing post-surgical option, NSAIDs, antidepressants, anticonvulsants, and even topical local anesthetics. Can you really use lidocaine patches in that many ways?
Let’s be honest. We’re a little desperate. (Ahem, not that medications aren’t sometimes necessary, but perhaps we should be asking ourselves why our population suffers from so much pain and why medication is often the first thing we reach for to treat it… but that’s a discussion for another day.)
So the pharma world answered. Obviously not entirely altruistically because there’s always a dollar sign hanging somewhere in the equation, but they have delivered a novel, first-in-class, non-opioid analgesic with the approval of suzetrigine (Journavx). Given this has the potential for significant uptake (and really, how often do we get new classes of medications?!), we figured it was worth a new drug approval post. Let’s dive in.
What is suzetrigine (Journavx)?
Ok, we have to talk about the elephant in the room. What kind of market research led to this v next to an x, and how are we supposed to pronounce it? Apparently, word on the street is that it’s pronounced “jur-na-vix.” Cute, mmm.
Now that we have that out of the way, we can just call it suzetrigine (which also makes me wonder if the developing scientist named it after his lifetime love, Suzie, or something). But I digress. On to the good stuff now.
Not that pharmacy suffixes always lead you down the right path (because no, metronidazole is not an azole antifungal…whyyyy), but in the case of suzetrigine, it kind of works. Suzetrigine sounds like lamotrigine, right? Well, they both work on voltage-gated sodium channels. But don’t take the analogy any further, or you might end up on that wrong path again, k?
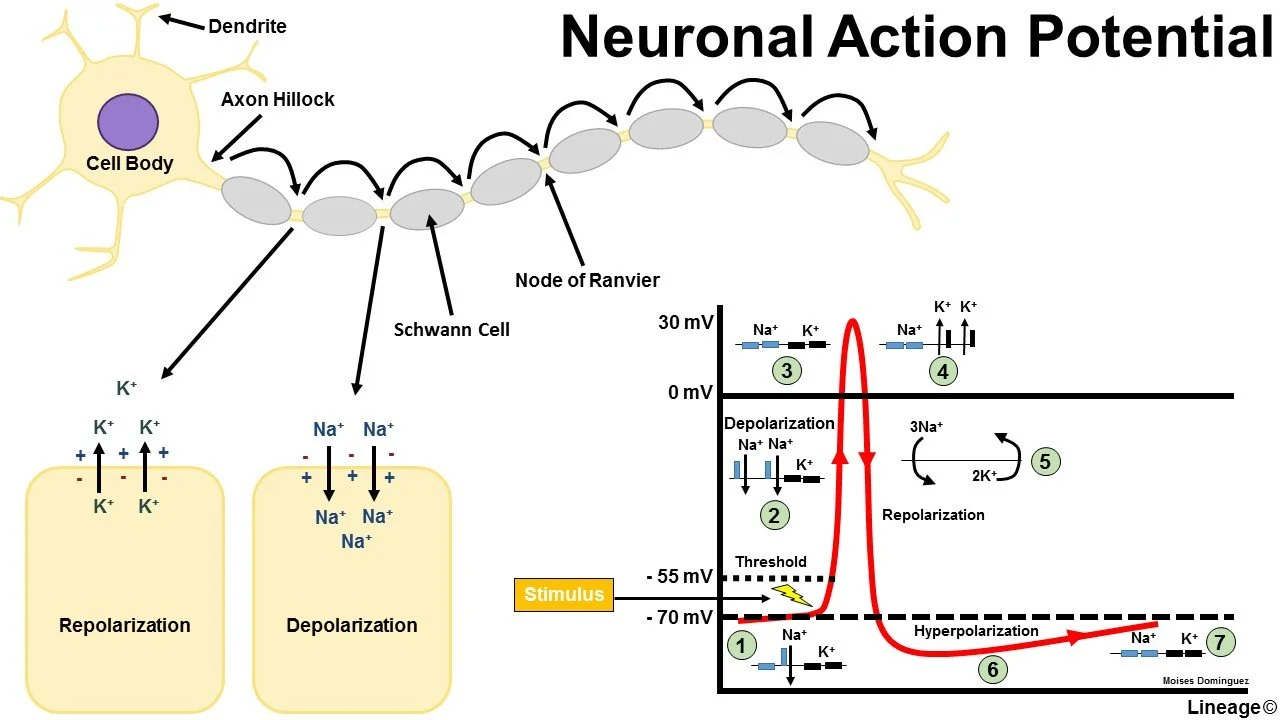
Let’s take another little detour to physiology. In order to understand how suzetrigine works and what makes it novel, we need to bring back our old friend, the action potential.
How do nerves propagate signals?
Nerves conduct messages, including pain signals, by producing and propagating action potentials. Action potentials are generated by the movement of ions in and out of ion channels, and they follow a specific pattern:
Depolarization,
Repolarization, and
Hyperpolarization.
Each neuron has a resting potential, which depends on its specific mix of sodium, potassium, and chloride permeabilities. This is the chill state, the status quo. Each neuron also has a threshold potential, which is the mix of charges necessary to “detonate” the neuron and create an action potential. Neurons get from their resting potential to their threshold potential by way of a stimulus, be it pain, light, smell, whatever. Something stimulates that nerve cell. Sometimes that stimulation is enough to reach the threshold potential, and sometimes it’s not.
(Image)
It’s like if you’re binging Netflix at home on a Thursday night, and you hear something that sounds like fireworks outside. Is it enough to get you to put down your bowl of popcorn and get off to the couch to check out the show (threshold potential reached, depolarization executed), or are you just too busy being a bum to even look (not enough of a stimulus to depolarize)…
Mmmhmm, we’ve all been that bum. No judgment.
Also, there is no in between. There’s no pausing the show to listen for a second. You either get up, or you don’t. Same with neurons. There’s either depolarization or not. All or nothing. No halfsies.
So let’s assume you’re not a total bum, and depolarization occurs. You get off the couch. With depolarization comes the influx of sodium ions from outside to inside the nerve cell through voltage-gated sodium channels. Then, this process peaks, the sodium channels inactivate and close, and repolarization begins, which is executed by the efflux of potassium from inside to outside the neuron. That cell’s potential decreases back to its resting state.
But does it stop there? Nope.
In an effort to prevent that cell from detonating again immediately, it actually overshoots the resting potential and hyperpolarizes so that it would take MORE than the normal stimulus to detonate again. This is a built in check and balance so that the action potential can’t go back the way it came. It keeps the message propagating in the right direction. So freakin’ cool.
Check it all out in the figure below:
(Image)
Now that we’ve reviewed how an action potential works and the role of the sodium channel in depolarization, let’s dig a little deeper into these special proteins. Voltage-gated sodium channels (Nav) come in 9 different varieties, Nav1.1 to Nav1.9. They’re present not only in the nervous system that we’re talking about with pain signals today, but also in cardiac, smooth muscle, and skeletal tissues.
Think about those local anesthetics and class I antiarrhythmics, e.g., lidocaine, flecainide, propafenone, quinidine. We know them as sodium channel blockers. What about the anti-epileptic phenytoin, which we also know as a sodium channel blocker… We’ve already mentioned lamotrigine as a sodium channel blocker as well. How are all of these very diverse medications sodium channel blockers, and yet, we’re certainly not using phenytoin for analgesia?
It comes down to those subtypes of Nav, as well as each medication’s specificity for the different types of Nav (versus sometimes other types of ion channels). These differences are what give each medication its profile - both therapeutically and in terms of adverse effects.
Digging a Little Deeper on Suzetrigine Characteristics
Alright, now we can return to the actual topic of this post, suzetrigine. What makes suzetrigine novel is that it is highly specific for blocking just one subtype of Nav - the Nav1.8. It just so happens that this particular Nav is involved in propagating pain signals in the periphery ONLY. It is not present in the CNS (unlike other types of Nav), thereby theoretically avoiding issues with CNS depression and with addiction and abuse.
Pretty slick concept, right? Very much a designer item. We’ll talk about how this designer item measured up in studies in a few, but first, some nuts and bolts:
At this point, we’ve got the mechanism, novelty, and nuts and bolts of suzetrigine under our belts. It’s time for The Question. Exactly how well does this designer drug work?
Clinical Studies of Suzetrigine
So unfortunately, the phase 3 clinical data for suzetrigine’s efficacy hasn’t yet been published. Vertex Pharmaceuticals, its developer, presented data in October 2024 at the annual meeting of the American Society of Anesthesiologists. But we can’t go full journal club yet due to lack of details.
Here’s what we do know, however…
Suzetrigine was evaluated in two separate phase 3 clinical studies, NAVIGATE-1 and NAVIGATE-2. To make your life a little easier (this is tl;dr after all), here’s a handy chart of some highlights:
Sorry, you might have to view this on your actual computer rather than your phone…
I know Bridget Jones is British not American, but the image disconnect still stands. (Image)
Of course, as always, there are pros and cons to every clinical trial. From what I can tell without the full publication, there are some limitations to these studies. First, the included populations consist largely of white females. They were investigating pain relief after tummy tucks and bunion removals, which due to our body image expectations (‘Murica!), generally involve females rather than males. Minority ethnicities were also underrepresented. Suzetrigine is supposed to be pain relief for any moderate to severe acute pain, but I’m not convinced their study populations are actually representative of that goal...
Second, the duration of follow up was (I’m sorry to say) incredibly short. These phase 3 trials only followed a 48 hour post-op course, and even though there is a 3rd open label trial that assessed safety of suzetrigine over 14 days, that is still remarkably abbreviated when you consider that this medication is approved for use up to 14 days. So what happens after that? Lab abnormalities…return of pain…I don’t know. Do you?
Third, the comparator opioid arm was limited to low dose hydrocodone/APAP 5/325 PO q6h. Even with this low dose, suzetrigine still couldn’t pull out a win in the bunionectomy trial (unless they also included ibuprofen use, not shown above). So what happens after more extensive surgeries or patients with higher pain at baseline? Not that I want to stick every post-op patient on oxycodone, but can we really consider this medication to be a replacement for our current opioid pain medication options?
Fourth, the only rescue medication allowed was ibuprofen. For anyone who’s seen the list of PRNs available post-op, you know this often isn’t representative of the real world. So how would having the usual gamut of PRNs available affect efficacy numbers? Also, how are we supposed to assess the opioid-sparing possibilities of suzetrigine if an NSAID is the only PRN available?
Finally, again because of the short follow up in the studies, we don’t have any information about how suzetrigine might impact how many people transition from acute to chronic pain. How might suzetrigine affect those numbers? Isn’t that really one of the huge goals that has come out of the opioid crisis - not to become dependent on pain medication chronically?
Sigh. The FDA granted suzetrigine’s application Breakthrough Therapy, Fast Track and Priority Review designations, so I’m thinking they were quite excited to see a selective, non-opioid pain medication option. But there are so many more questions.
Keeping these unanswered questions in mind, let’s turn for a hot minute to cost. Thirty tabs of hydrocodone/APAP 5/325 (figure about a week’s supply) costs about $10-20 without insurance. One tab of suzetrigine 50mg is set at $15.50 without insurance. Soooo yeahhhh. Now who knows how insurers will view this non-opioid option. Perhaps they’ll consider it worthwhile to avoid chronic opioid issues. Only time will tell.
The manufacturer is also offering patient support programs, including a coupon for BOTH commercial and government insured patients, which is highly unusual. Patients may pay “as little as $30 per prescription.” Maybe the cost difference won’t be as large as it seems? But there are terms and conditions on this coupon too, so it could get tricky.
I also want to know how their coupon terms and conditions already talk about 30 day supplies when the medication is only approved for 14 days of use… Interesting, right. #offlabeluse
The tl;dr of Suzetrigine (Journavx)
So there you have it. The first selective voltage-gated sodium channel blocker for acute moderate to severe pain. Suzetrigine blocks the transmission of pain signals from the peripheral nervous system by blocking the Nav1.8 channel. While the available evidence certainly has its ups, downs, and unanswered questions, it is always really fascinating to see how medications can be developed to meet a very specific need. It will be interesting to see the uptake and integration of suzetrigine into medication protocols and patient care. So stay tuned, and happy new drug learning!