Hyperkalemia: An Overview for Pharmacists
Editor's Note: Please give a warm and fuzzy tl;dr welcome to Sophia Pathan, who was kind enough to write the post you're about to read. Sophia is a P4 student from the Ernest Mario School of Pharmacy at Rutgers University in New Jersey. She is interested in Infectious Diseases and Critical Care and hopes to attain a PGY1 residency and get further involved in clinical pharmacy and academia.
Sophia, you did a great job with this. Thanks! I'm gonna shut up now :)
Hyperkalemia
It’s arguably one of the more important electrolyte disorders, since a potassium level that’s out of whack can quickly lead to fatal heart arrhythmias.
In fact, potassium used to be (and is still) part of the cocktail that makes up the lethal injection. Because the stakes are so high with elevated potassium ("patient death" is sort of a big deal), we need to bring our A game when studying it.
With that said, let’s dig into the world of hyperkalemia.
Hyperkalemia Background
Let’s take a step back. What is potassium? Why do we need it?
You might remember learning about the Na+-K+-ATPase pump (cue the flashbacks to bio and biochem), because one of potassium’s major roles in the body is to help form the electrical gradient across our cell membranes.
Potassium transfer regulates cell polarization, making the resting membrane potential negative. Basically, the inside of our cells are negatively charged compared to the outside of our cells, and that plays a role in action potentials causing nerves to fire.
So, among other things, potassium affects neurotransmission, muscle contractions, and heart contractions. We also need potassium for cell metabolism and protein synthesis, growth, and anabolic activity.
We need a good amount of potassium to do all that. But what is a "good amount" of potassium?
Well, normal serum potassium levels are about 3.5 to 5 mEq/L. But this only accounts for around 2% of potassium, since most potassium in the body is intracellular (i.e. inside the cells).
If you want to get really fancy and you’re willing to do some math, the total store of potassium in your body is based on body weight. If you multiply your patient’s body weight in kg by 50 mEq, you’ll know the actual amount of potassium in the body, which is usually above 3000 mEq.
We maintain potassium levels in our body through dietary intake (meats have a ton of it) and renal excretion (watch out for patients with kidney disease!). All in all, our bodies (when healthy) do a pretty good job of keeping serum potassium within the 3.5 to 5 mEq/L range.
When potassium levels aren’t maintained properly (either through too much intake or too little excretion), you end up with hyperkalemia.
There isn’t a universally accepted definition of what it means to have mild vs moderate vs severe hyperkalemia. But as a general guideline;
Mild hyperkalemia - 5.5 to 6.5 mEq/L
Moderate hyperkalemia - 6.5 to 7.5 mEq/L
Sever hyperkalemia - anything greater than 7.5 mEq/L
(This differs slightly from the chart below, but the definition depends on your hospital site, how stringent you are in treating patients, etc...)
(Source)
So although elevated potassium is a big deal and needs to be treated urgently, a potassium level of 7 doesn’t spell instant death for your patient. There’s plenty we can do as pharmacists to manage hyperkalemia (and prevent it from occurring in the first place).
It all starts with lab monitoring and symptom recognition. The lab monitoring is easy. You draw either a BMP or a CMP and you send it off to the lab to get your patient’s potassium level.
Recognizing the symptoms of hyperkalemia is a little trickier. Your patient might show a number of symptoms. Or none at all. The most common symptoms are muscle weakness or paralysis, palpitations, and syncope, followed by cardiac conduction abnormalities and arrhythmias.
Two disease states that result from tissue breakdown (rhabdomyolysis and tumor lysis syndrome) can also cause hyperkalemia and the above symptoms.
Obviously if your patient has hyperkalemia, you probably need to do something to fix it. But if the patient is symptomatic, or if serum potassium is > 7, or if you notice ECG changes, then you need to be in full on crisis mode and doing something about it STAT.
There are a whole slew of a different reasons that your patient might have hyperkalemia.
Chronic kidney disease (generally in stages 4-5)
Addison’s Disease (adrenal failure)
Rhabdomyolysis (the breakdown of muscles that will lead to breaking your cells, spitting out all of that intracellular potassium)
And of course there are plenty of drugs that can cause or contribute to hyperkalemia.
Potassium (definitely a hard one to remember)
ARBs
Beta-blockers
Digoxin
Heparin (heparin is a slight aldosterone antagonist, which means it can act kinda like spironolactone and increase potassium levels)
NSAIDs (they can cause AKI)
Potassium-sparing diuretics
And a whole bunch of other drugs that would make this article even longer than it already is
The most common cause hyperkalemia is acute kidney injury (AKI), but hyperkalemia tends to be multifactorial. The AKI could be drug-induced, for example. Or the patient has AKI on top of their chronic kidney disease (CKD) while they’re taking an ACE inhibitor for their heart failure.
Hyperkalemia Treatment
So we need to treat the elevated potassium. That's a given. But it’s important to know what caused your patient’s hyperkalemia. If you don’t fix the underlying problem, you're just putting a bandaid over the problem and it will return.
That being said, we need to prioritize. Even something severe as an AKI can wait if you're patient is in a fatal heart arrhythmia and their potassium is at 8 mEq/L.
As we'll learn in a minute, cardiac stabilization takes treatment precedent. Then we talk about what to actually do with all that potassium in the serum (not to mention figuring out what caused the problem in the first place). But let's not get ahead of ourselves.
First things first, what are our goals? Obviously, we want to decrease potassium levels. They’re high (hence hyper-kalemia) and logic dictates we want them back down to normal serum levels.
Notice that I’m saying “serum” (this is a fancy word for “blood”). Remember from earlier that most of the potassium in your body lives inside the cell. The symptoms of hyperkalemia are from having too much potassium outside the cell. We can lower serum potassium one of two ways:
Shift potassium into the cells
Remove potassium from the body (i.e. enhancing elimination)
And again, if the patient is having ECG abnormalities or is in arrhythmia, we’re also going to want to do something to fix that. We’ll get to that in a second.
First, let’s talk about some monitoring parameters for the hyperkalemic patient.
You’re obviously going to want to monitor potassium levels throughout this process. If it hasn't clicked that you need keep an eye on the potassium level in this patient population, I don’t know what to tell you.
Check every 1 to 2 hours, while the patient is symptomatic. Then once the level stabilizes you can back off to every 6 – 8 hours and then daily. And don’t forget to watch those ECGs!
Finally, it’s a good idea to check daily weights, fluid intake, and urine output to assess fluid balance (that will make more sense when we look at some of the treatments below).
Now, before getting into the nitty gritty, there’s a quick preface to mention about the treatment of hyperkalemia.
Get rid of any meds/fluids that can make it worse.
This seems obvious when you point it out, but in the midst of an acute situation, it’s surprisingly easy to forget. So stop any fluids with potassium in them, and any drug that can increase potassium levels (ACE inhibitors, ARBs, aldosterone antagonists). Also don’t forget about drugs like NSAIDs that can impair potassium excretion.
Don't make a bad situation even worse. And don't complicate the already difficult job of managing hyperkalemia.
Alright, with that out of the way, let’s move on.
Step 1: Stabilize the Cardiomyocyte Membrane
So what exactly is “stabilizing cardiomyocyte membranes?” Am I just making up words?
Well, no. I’m not. Arrhythmias are the biggest causes of mortality in hyperkalemia patients, and if we can stabilize the heart muscles from contracting too much and too quickly, then we’re keeping the patient alive. Once that's stable, we can actually fix the potassium problem.
Never thought you'd have to see this again, did you? (Source)
Increased potassium changes the heart’s resting membrane potential.
When you look at the action potential of the cardiac myocyte, potassium exerts its main effects on Phase 2 and Phase 3.
At Phase 2, potassium leaves the cell while calcium enters it, creating the plateau phase (it plateaus because the positive charges leaving the inside of the cell with potassium are replaced with the positive charges coming into the cell with calcium).
During Phase 3, potassium continues to leave but the calcium channels close. Now there are more positive charges leaving the inside of the cell (making it overall more negative) and the cell's resting negative membrane potential is restored (Phase 4).
When a patient has hyperkalemia, several things happen. For starters, the resting membrane potential is made less negative (i.e. it takes less work for an action potential to fire).
And on top of that (for reasons that are poorly understood), hyperkalemia leads to even greater potassium loss from the cell (and more quickly).
This increased potassium efflux, makes the Phase 2 and Phase 3 portions of the graph above have a more downward, negative slope. This manifests as a shortened repolarization time (where you reach the cell’s resting potential more quickly), so the cell can fire off action potentials sooner.
So you've got a scenario where it's easier for the heart to contract...and it can contract more rapidly. This is the root cause of the arrhythmias seen with hyperkalemia.
And our goal is to keep that from happening.
We give calcium to stabilize the cardiomyocyte during hyperkalemia. Calcium helps to balance out the change in membrane potential seen when potassium leaves the cell (see Phase 2 above). This blunts the "hyperexcitable" state of the heart (leading to lower likelihood of arrhythmia).
Let's put that another way. Remember that in hyperkalemia, the depolarization threshold that is required for an action to propagate is reduced. It's "easier" for an action potential to fire. Giving calcium re-establishes the normal threshold, making it less likely the patient will experience rogue action potentials firing and causing arrhythmia.
That’s still. . . pretty confusing. Example time!
Pictures are worth 1000 words (and about 15 mV). (RMP = Resting Membrane Potential). (Source)
Let’s say, normally the resting membrane potential of a cardiac myocyte is -90 mV, and the threshold potential is -75 mV before the heart will beat.
This means that (normally) there needs to be 15 mV of depolarization (making the cell less negative) to make the membrane potential reach the threshold and fire.
This causes the heart to beat.
In hyperkalemia the resting potential is increased. So for this example we’ll say it’s at -80 mV instead of -90 mV. (Don’t forget how negative numbers work!).
That means only 5 mV of depolarization is needed before the action potential fires (and the heart beats). It's a lot easier to get to 5 mV than 15 mV, so more action potentials fire (causing more heart beats).
When you give a patient calcium, the threshold potential (don't confuse it with the resting potential) is increased.
In this example, we’ll say that the threshold potential of -75 mV is increased to -65 mV. Remember that hyperkalemia increased our resting potential from -90 mV to -80 mV.
So the heart has to get from -80 mV to -65 mV to fire an action potential and beat. This is a difference of 15 mV of depolarization, which happens to the same amount needed during normal physiologic conditions.
So, you’re basically giving calcium to prevent the heart from freaking out and going into arrhythmia.
(If that was still confusing or you want more details because hyperkalemia is just THAT good, this article explains everything very well.)
To stabilize the cardiac membrane, there are two types of calcium you can use.
Calcium gluconate (preferred)
Calcium chloride
Calcium gluconate is given as a 1 gram IV push over about 7 minutes. You’re more likely to see this in practice than the other option, calcium chloride (500-1000 mg IV push over 2-5 minutes).
Remember this chart? (Source)
Why do we prefer calcium gluconate? Well, for starters, calcium chloride is three times as potent as calcium gluconate.
It's also an irritant when given IV and will burn your patient’s veins something fierce.
To get around this problem we administer calcium chloride through a central line. Good luck getting that placed (if your patient doesn’t have one already) in the middle of a hyperkalemic emergency.
The one advantage of calcium chloride is that it can be administered more quickly than calcium gluconate (which can cause severe hypotension if pushed too fast).
So again, to recap. Let's remember that calcium does nothing to treat the underlying problem (elevated potassium). But it does buy you time. It will keep your patient stable enough while you diagnose and fix the potassium problem. In practice, it’s too risky to not give calcium.
Step 2: Intracellular Potassium Shift
Alright. So we’ve stabilized the myocardium (if the first dose of calcium gluconate doesn’t stabilize the EKG, go ahead and repeat it).
Now for the next step; actually lowering serum potassium levels. How do we do that? Like I said before, you can either shift the potassium into the cells, or remove it entirely from the body.
Let's start with shifting potassium into the cells. It works faster than potassium removal, so this will help keep us out of the danger zone while we buy ourselves more time.
There are a few ways to shift potassium into the cells:
Insulin
Beta-2 agonists
Bicarbonate
Your first choice in an emergent situation is usually insulin. It’s safe and works quickly (I’ll get to the how in a minute).
A typical dose of insulin for hyperkalemia is 5 – 10 units IV.
Of course, insulin is not so safe if your patient has low blood sugar (Spoiler alert: insulin lowers blood sugar). So IV dextrose (25 – 40 g dextrose in 50% solution) is usually given alongside the insulin to balance that out.
If your patient’s blood glucose is > 250 mg/dL, you don't need to worry about giving the dextrose because they've got enough of a blood sugar buffer to handle 10 units of insulin.
You can also use beta-2 agonists (such as albuterol) for hyperkalemia. They lower serum potassium (actually via the same mechanism as insulin), but they take a lot longer.
You could consider albuterol adjunctive therapy for hyperkalemia (or as an option to use when time is not a factor). The usual dose is albuterol 20 mg via nebulizer.
So, what is this magical mechanism that insulin and albuterol BOTH use to lower serum potassium?
The picture above is of the skeletal muscle, but the idea is the same. You can see that insulin and a B2 agonist will both (via a slightly different intracellular pathway) increase Na+-K+-ATPase pump activity, which pulls potassium into the cells.
All they're doing is increasing the activity of Na+-K+-ATPas (the same pump I mentioned earlier that establishes your heart's resting membrane potential).
Remember that most potassium lives inside the cells of your body. In hyperkalemia, there's a lot of potassium outside of the cells. By activating the Na+-K+-ATPase pump, you’re moving some of that extracellular potassium into the cells again.
Again, you’re not actually decreasing potassium levels, you're just sort of sweeping the problem under the rug to buy yourself some more time. This will help you prevent the arrhythmias and other cardiac problems I talked about earlier.
The total amount of potassium in the body (that 50 mEq x weight in kg) is exactly the same, and, eventually, you’re going to have to deal with the extra potassium (don't worry, I'll get to that in a minute).
If you’re really interested in the mechanism of how beta-2 agonists and insulin shift potassium to the inside of the cells: They both stimulate the Na+-K+-ATPase pump through different signaling pathways.
Insulin phosphorylates a substrate within the cell (IRS-1), with activates a kinase (PI3-K) to phosphorylate another kinase (aPKC), to insert Na+-K+-ATPase on the cell membrane.
Beta-2 agonists use cAMP and PKA signaling to phosphorylate ADP to ATP, which activates Na+-K+-ATPase to bring potassium into the cell.
(Source)
Another way to force potassium into the cells is with sodium bicarbonate.
How does that work?
Well, bicarb, you hopefully know, raises serum pH and makes your blood more basic.
Your body will try to compensate for this by decreasing pH back to our happy physiologic territory of 7.4. (Homeostasis!)
And it turns out that this process involves potassium. There are two main mechanisms that your body uses to acidify yourself, and both processes shift potassium into your cells.
So, we've given your patient bicarb. Their serum pH has increased, affecting homeostasis.
The first thing the body can do is increase H+/K+ exchange. The body wants to spit out protons (since they are what increase acidity), and it can do that by sucking potassium into the cells. Since your patient is hyperkalemic and there’s a ton of extra potassium floating around, this is a win-win situation.
The other way to decrease pH is through HCO3-/K+ transport. The cells will pull the bicarbonate out of the blood directly, and one of the necessary downstream effects is intake of potassium into the cells.
(Source)
Both of these mechanisms decrease serum potassium by shoving the potassium intracellularly until you have the chance to actually get rid of it.
You can give sodium bicarb 50-100 mEq IV over 5 minutes.
If you remember anything about your acid/base physiology, you really don't want to mess with your bodies pH. It affects so many physiologic processes (plus there are a ton of monitoring parameters with bicarb).
So unless the patient has a compelling reason (such as DKA or some other acidosis) you probably aren’t going to use bicarb as a first line agent.
Some of those monitoring parameters for bicarb are cellulitis, injection site extravasation, skin ulcer, tissue necrosis, and obviously blood pH (because if you give too much bicarb you can cause a metabolic alkalosis).
Insulin, albuterol, and sodium bicarb are great and all. But like I mentioned, none of this actually decreases serum potassium levels. We’ve pushed it into the cell, which gets us out of the immediate danger zone. . . but we’ve got to get rid of the potassium at some point. This brings us to the next treatment option:
Step 3: Remove Potassium From the Body
Kayexalate's mechanism of action.
Potassium-binders (also called “gastrointestinal cation exchangers” by the internet and by absolutely no one else) bind to potassium in the GI tract and cause your patient to poop it out.
The gold standard agent for potassium elimination is 15-30g of sodium polystyrene sulfonate (Kayexalate) PO daily.
You may also hear it go by the name “SPS” (check out the generic name to understand why).
Since Kayexalate relies on the digestive system (and digestion can take a decently long time), it takes longer to work than everything we've talked abut so far.
This is why you need to give the short-term insulin or bicarbonate to “hide” the potassium while the Kayexalate does its work. You have to do both. Direct elimination of potassium CANNOT be done alone in emergent situations.
Kayexalate exchanges the potassium with sodium, magnesium, and calcium. So be sure to keep an eye on those levels. When your patient’s blood is drawn for the potassium level monitoring, just ask for those levels too. Look at you, multi-tasking.
There’s also a fancy new (ish) drug called patiromer (Veltassa), which your patient will take as an 8.3g dose PO (it’s a packet that you dissolve in water) daily. It's supposed to be used as a chronic medication.
Similar to SPS, patiromer is a calcium exchanger. It’s supposed to be used in the chronic setting for patients taking ACEs or ARBs because (as I’m sure you remember) those carry a risk of hyperkalemia.
Patiromer’s package insert specifically says not to use it in emergencies, but in practice (at least in some institutions) it is used for acute hyperkalemia as well as chronic.
Considering how much newer (and more expensive) patiromer is, you’re probably going to use Kayexalate. But now you’ve got more drug trivia to file away for your next exam.
Another option to lower serum potassium is a loop diuretic (ex: furosemide 20 to 40 mg IV).
Remember that loops cause a decrease in basically every electrolyte. So as long as your patient's other electrolytes are stable (and he/she isn't dehydrated) they're actually a pretty good option.
Loops also take a little while to work, since they work by increasing potassium excretion through the urine. Also remember that in the acute setting, loop diuretics can cause/worsen a kidney injury (and the most common cause of hyperkalemia is renal dysfunction).
So if your patient has an AKI or is severely dehydrated, it's best to avoid loops. You can use loops if the patient only has mild kidney dysfunction.
If dehydration is a factor, you can also give fluids along with the loop diuretic. Just be aware that you’re giving fluid and a diuretic at the same time (and those are sort of opposing ideas). Again, use your clinical judgement.
Finally, while not technically a “pharmacy” thing, the last step for treating hyperkalemia is dialysis. I’m not touching that topic with a ten-foot pole, though. So just be aware that when all else fails, dialysis is your last resort.
Hyperkalemia Summary
Let's review everything, shall we?
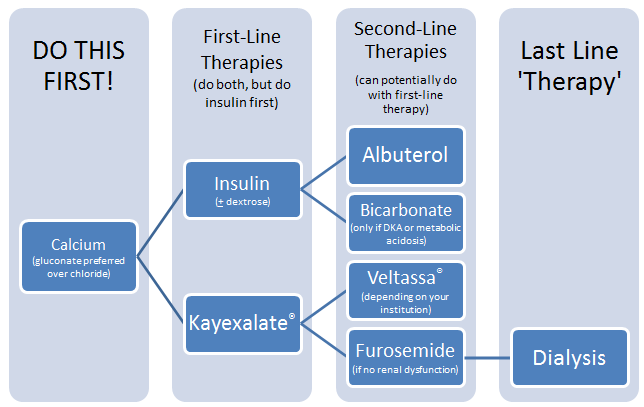
Hyperkalemia (when serum potassium levels >5 mEq/L) can lead to various cardiac abnormalities and death, so the first step in management is to stop/prevent arrhythmia.
First, you give calcium gluconate to stabilize the cardiomyocyte membranes. Next, give insulin (preferred) or a beta-2 agonist to shift potassium from out of the bloodstream and into the cells.
Don't forget to stop any medications that could be causing or worsening hyperkalemia.
After all of that, you can start actually removing excessive potassium from the body. Give a potassium binder (Kayexalate) or a loop diuretic (furosemide) to take the potassium out of the body entirely.
tl;dr: Give your patient calcium to keep them alive, then give insulin to shift potassium intracellularly and Kayexalate to remove excess potassium.
(Source: Crappy Microsoft Word skills)