Atrial Fibrillation: The Pharmacist's Survival Guide
Editor's Note: The following post was written by J. Nile Barnes, PharmD, BCPS, and Chelsey Roscoe, Doctor of Pharmacy Candidate 2018.
If you've been around tl;dr pharmacy long enough, you're probably familiar with Dr. Barnes. He wrote our series on Heart Failure (Part I, Part II, Part III). He's a Clinical Assistant Professor of Health Outcomes and Pharmacy Practice at the University of Texas at Austin College of Pharmacy. He's also a clinical pharmacist for an academic medical center. Prior to becoming a pharmacist, he spent several years as a paramedic. He's got hands on experience with most levels of medical practice and pharmacy education, and he's truly one of the best teachers I know.
Chelsey Roscoe is a P4 student from the University of Texas at Austin College of Pharmacy. During her P3 year, she served veterans as a Valor intern at the CTVHCS anticoagulation clinic. She is interested in Ambulatory Care, Psychiatry, Oncology and Infectious Disease Pharmacy. She plans to pursue a PGY1 residency in 2018 where she can be fully involved in the patient care process.
Stick around to the end, and you'll be rewarded with instructions for dosing diltiazem, digoxin, and amiodarone in afib patients.
I'll let Nile and Chelsey take it away...
The Cardiac Cycle
The thought of dysrhythmias is enough to cause a dysrhythmia in the hearts of most healthcare students. This is understandable, as they can be complicated at times.
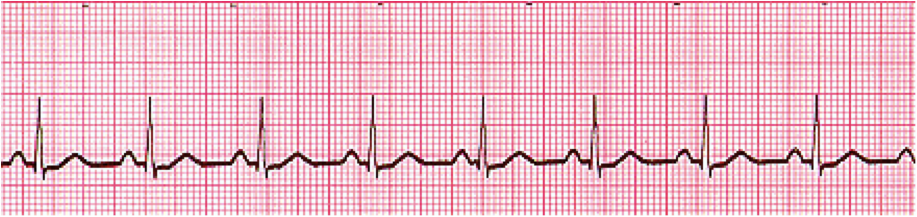
The most common sustained arrhythmia is Atrial Fibrillation or Afib or AF. Afib is seen in the ECG (lead II) below.
An ECG of Afib (Image)
To understand atrial fibrillation, we need to understand the basics of the cardiac cycle and the ECG (electrocardiogram). The animated gif below shows how the usual cardiac cycle correlates with the ECG.
And this image (to the right) lists all the segments and intervals in a normal ECG (note that U waves are included, they are poorly understood, but may represent “after-depolarizations” which may trigger some arrhythmias).
Let's review the various segments of a normal ECG and how these correlate to the normal cardiac cycle.
The P wave on the ECG marks the beginning of the cardiac cycle. It represents the depolarization of the atrial tissue due to a signal from the SA node.
The SA node is the king of the heart, the natural pacemaker that determines how fast the heart can beat.
When the impulse arrives at the AV node (another natural pacemaker,) the AV node acts like a cross-walk guard, pausing the electrical signal to allow the atria time to depolarize and have the mechanical contraction necessary to pump the blood into the ventricles. This delay in the electrical signal is represented by the PR interval on the ECG.
After this, the AV node depolarizes and the electrical impulse travels down the Bundle of His and out the Purkinje fibers to the ventricular muscle tissue. This is represented by the QRS complex in the ECG.
After the QRS complex, the T wave occurs (which represents repolarization of the AV node and ventricles).
BTW, there is a tiny atrial Tp wave (which represents repolarization of the atria,) but it is so tiny that the QRS dwarfs it. This signal is smaller because the muscle fibers of the atria provide a much smaller contraction than the ventricle (which makes sense because the atria only needs enough force to pump blood to the ventricle, whereas the ventricle must produce enough force to pump blood to the rest of the body.)
The QT interval is the combination of depolarization (QRS wave) and repolarization of the ventricle (T wave.) We worry about prolongation of the QT interval with many of our drugs, including antiarrhythmics, antiemetics, antipsychotics, and some antibiotics.
Prolongation of the QT interval can increase a patient’s risk for Torsades de Pointes, a polymorphic ventricular tachycardia which can lead to hemodynamic instability and sudden cardiac death. (See below).
It looks sort of like a party streamer. But Torsades de Pointes is a medical emergency. (Image)
As a quick side note, the QT interval can still be measured in afib, but it is almost impossible to measure it in atrial flutter. We won't cover atrial flutter in this post; just know that the "flutter waves" almost completely obscure the T wave--making it difficult to measure QT.
After repolarization of the ventricle (the T wave), the cardiac cycle repeats itself.
The body usually adjusts the heart rate by adjusting the sympathetic and parasympathetic inputs to the SA and AV nodes. Sympathetic (adrenergic) stimulation will speed the heart rate while parasympathetic (vagal) stimulation will slow the heart rate.
Alright, that covers the basics of the cardiac cycle and ECGs. Let's move on to the meat of this post, atrial fibrillation.
Atrial Fibrillation Pathophysiology
There are several causes of atrial fibrillation, but since that is not the point of this post I will refer you to J Clin Invest. 2011;121(8):2955-2968 to read more if you are interested. This graphic from the article summarizes most of the causes.
It's important to cover the pathophysiology to explain a disease state, so let’s talk about the hemodynamic changes seen in Afib.
The hemodynamic changes are the consequence of King SA Node of the House of Atria losing reign of his atrial kingdom.
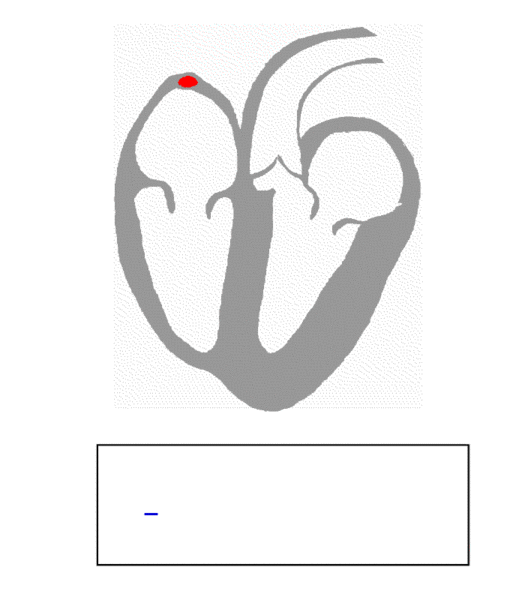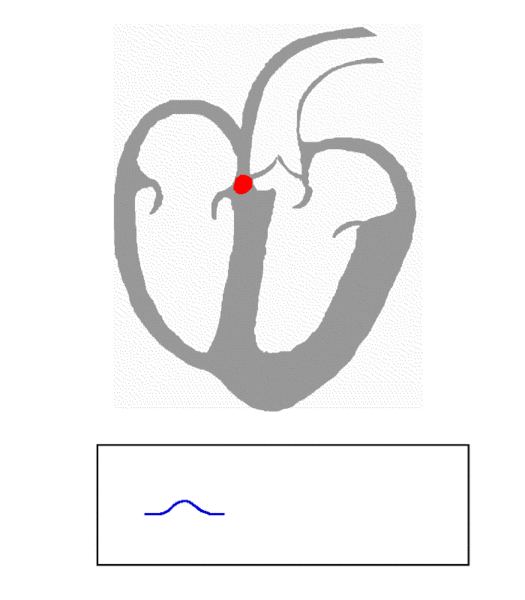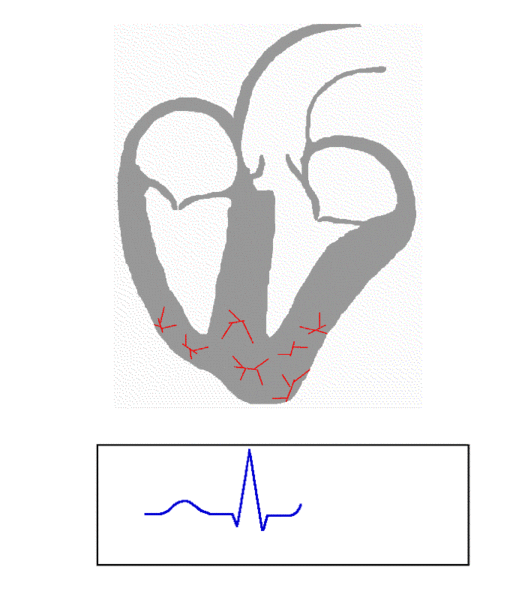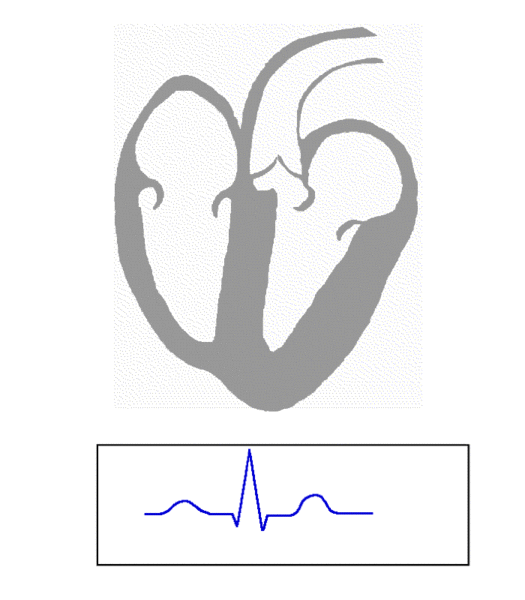
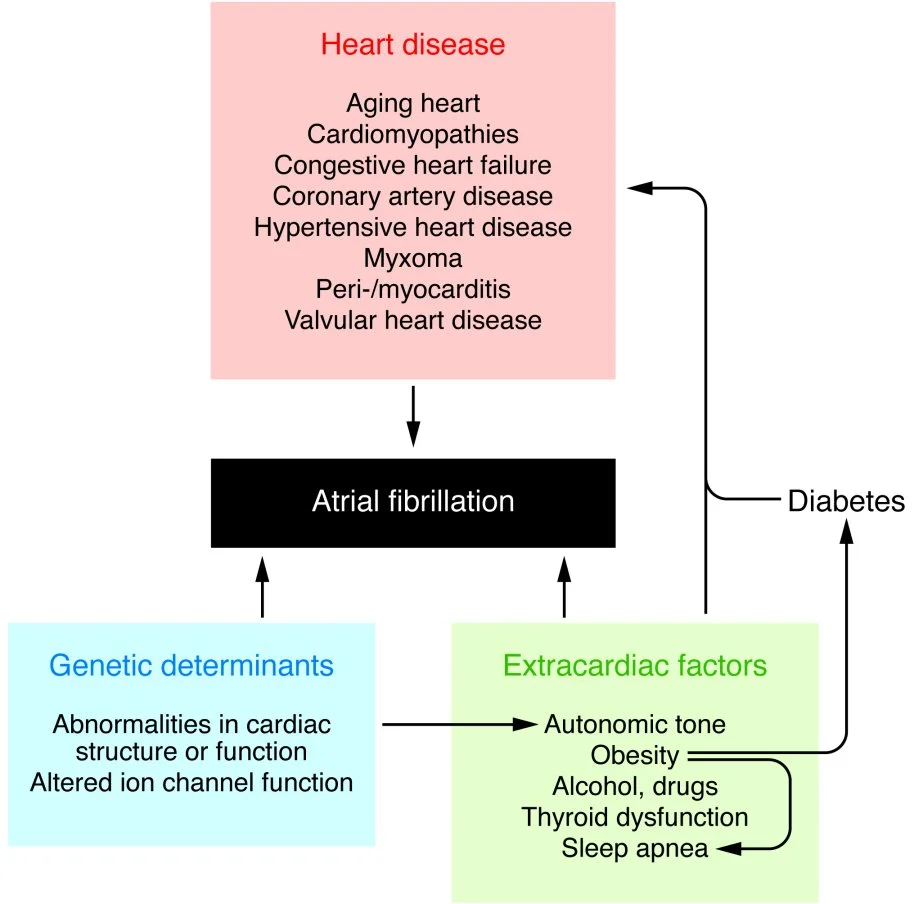
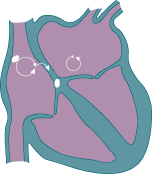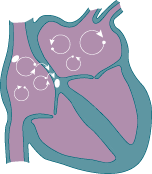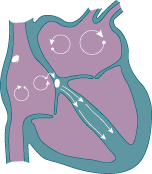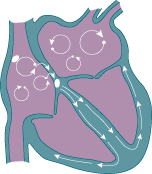
As a result of this usurpment, the atria does not have organized electrical activity; instead, the atria quivers, like jello.
Rather than King SA Node initiating an organized depolarization, multiple usurpers (sites in the atria) attempt to initiate the impulse. The result of this disorganized contraction is 500-600 fibrillatory waves (per minute) propagating across the atria with only a few of the impulses resulting in actual contraction of the ventricle (the QRS wave.)
The upshot here is that if the ventricles depolarize too fast, they will not be able to fill completely.
Electrical activity in afib. (Image)
Remember that delay on the ECG in between the P wave and the QRS called the PR interval? That was the time for the atria to empty into the ventricles.
Most people think the atria completely fill the empty ventricles, but that is not true.
Most ventricular filling (~70%) is passive movement from venous return (return of blood from the rest of the body to the heart). The atria then “top off” the tank by stuffing a little more blood into them (roughly 30% more). This extra is sometimes referred to as the “atrial kick.” In afib, the atrial kick is lost. This means the patient will often experience fatigue, exercise intolerance, weakness and dyspnea—although not everyone will have these symptoms.
Probably the most important consequence of afib is clotting and the possible resultant embolic events (i.e. stroke.)
Since the atria are no longer squeezing and relaxing in a concerted motion but are instead quivering, blood is not ejected from the atria with each beat. The blood primarily drains through the atria into the ventricles passively, as mentioned above, but some will hang out along the walls. This blood can adhere to the walls and form clots (the so called mural thrombus).
Jeremiah the Innocent (Image)
Speaking of murals, here is one of our favorites, not far from the Austin campus of the University of Texas: Jeremiah the Innocent.
Unlike the stationary Jeremiah, mural thrombi may break off and be pumped away.
If you look at the anatomy of the Aorta, you should realize that it’s time to be scared.
A coronary, a cerebral, and a subclavian walk into a bar... (Image)
The first vessels coming off of the aorta are the right and left coronary arteries, if a clot gets pumped into one of these, the patient will likely have a myocardial infarction (a heart attack).
Other vessels a clot can travel through (with terrible consequences) are the left and right common carotid arteries.
These two carotid arteries then bifurcate into the external and internal carotids. The internal carotids carry blood to the brain. Clots that break off and travel from the heart toward the brain are emboli. When these emboli lodge in the progressively smaller vessels in the brain, the result is an embolic stroke or CVA (cerebral vascular attack).
BTW, clots can also go down the subclavian arteries to the arms and get stuck too.
I once had a patient with all three events happening at once! He had a right-sided myocardial infarction (clot in the right coronary artery), another clot in the left subclavian artery that got stuck in the brachial artery and a right middle cerebral artery stroke. This was a very bad day for this gentleman (and very unusual too!).
Atrial Fibrillation Treatment and Clinical Pearls
Ok, so we have covered the pathophysiology and the consequences. Now, what can we do to protect the hearts and minds of millions?
Most of us would want to fix the underlying problem—by converting the patient back to Normal Sinus Rhythm (NSR.)
This would mean restoring the rightful monarch (King SA Node) as the sole electrical signal of the heart to allow for a peaceful kingdom with normal atrial contraction.
Normal Sinus Rhythm (NSR) (Image)
This sounds great but is harder to accomplish than it sounds. First we need to see how symptomatic the patient is, then determine how long he/she has been in afib. We also need to find out if there's been an attempt to convert to NSR in the past.
There is a wide spectrum of how afib patients may present. Some patients have afib for a while without knowing it. They might not have the bothersome symptoms of dyspnea, fatigue, etc. On the other hand, some patients with afib refractory to treatment (permanent afib) may be unable to convert back to NSR.
In some patients who have recently experienced significant trauma or infection, afib may be temporary (Lone afib) and so conversion to NSR will occur naturally on its own (usually within 24 hours of stabilization). It is often unnecessary to convert these patients.
There is a phrase you should know:
Atrial Fibrillation begets Atrial Fibrillation.
There was even a study in goats proving this. I don’t generally think of my patients as goats, (but this does remind me of the really bad movie with George Clooney).
Speaking of Permanent afib and Lone afib, there are a few terms we have to classify atrial fibrillation (AF):
Lone AF – AF sometimes develops in younger individuals with significant trauma without any evident cardiac or other disease. These patients have a very favorable prognosis compared with other AF patients. Read more here: Int J Clin Pract. 2014;68(4):418-433.
Paroxysmal AF – Afib that occurs sometimes and then stops without intervention. The heart returns to normal rhythm within 7 days.
Persistent AF – Afib that does not stop by itself within 7 days, but can be converted.
Permanent AF – Afib that cannot be corrected despite care and/or the provider and patient agree not to try any more treatments to convert to NSR.
Post-Op AF – Post-operative (usually lung or heart) atrial fibrillation (POAF) Read more here: J Thorac Cardiovasc Surg 2014; 148:772-791.
Valvular AF – Afib caused by a valve problem, such as a leaky or tight valve.
Non-valvular AF – The structure of the heart and the valves are otherwise okay but the patient has Afib.
Controlled AF – AF with ventricular rates < 100 BPM
Uncontrolled AF – AF with ventricular rates > 100 BPM
AF with a Rapid Ventricular Rate (or RVR)– AF with a ventricular rate > 130 and signs of decreased perfusion.
If anything, the above list should teach you to never call any afib “PAF”, because no one will know which type you are talking about.
Simple atrial fibrillation with no signs and symptoms is NOT an emergency. Yes, the patient needs care, but don’t panic.
If the patient presents with atrial fibrillation with a rapid ventricular response (heart rate >130 BPM) plus symptoms (shortness of breath, fatigue, etc...), then you should be worried. (But still, don’t panic!)
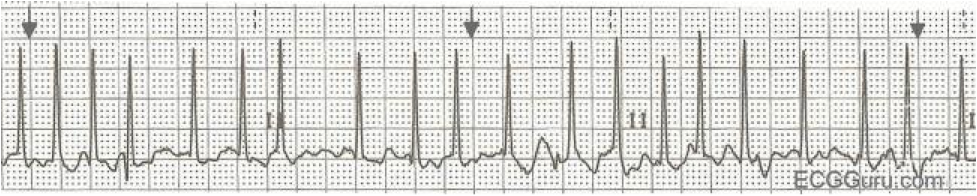
Afib with RVR (Image)
The ECG above shows Afib with RVR (about 180 BPM). I suspect this patient would be symptomatic. If this particular patient is in extremis, (e.g. acute heart failure or hypoxic), then we would electrically cardiovert him/her (we won't be covering that in detail today).
Let’s say that despite this ECG reading, the patient is not THAT ill clinically – this would be considered “urgent” instead of emergency.
In this case, we can either slow the ventricular rate or chemically (i.e. pharmacologically) cardiovert to NSR. Both treatments have the same long-term outcomes and rate control is usually MUCH easier (see the AFFIRM trial). So, let’s slow the rate.
Rate Control vs Rhythm Control is often hotly debated. Rate control means slowing down the Afib, usually by slowing the AV node so that fewer impulses get through. Rhythm control means converting the rhythm from Afib to Sinus Rhythm.
Frankly, rate control is easier and for most patients it is all you need to do.
According to the ACC/AHA guidelines, Calcium Channel Blockers (CCBs) and Beta Blockers (BBs) are the preferred agents for acute control of heart rate in patients with atrial fibrillation.
These are the agents you'll use in the urgent/emergent setting.
We have a few choices, all of which work by slowing AV nodal conduction in the heart and can be given IV. We'll list them out by class and give some clinical pearls for each.
Rate Control Agents for Acute Afib
1. Non-dihydropyridine Calcium Channel Blockers
This includes diltiazem (preferred) or verapamil. Here are some things to consider before using CCBs:
Do not use CCBs if the patient has heart failure w/ Ejection Fraction <40! Non-dihydropyridine calcium channel blockers cause decreased force of heart contraction (negative inotropic effects) which can further reduce someone’s ejection fraction.
There's also a risk of edema (though it's thought to occur less with diltiazem/verapamil than other CCBs)
Both diltiazem and verapamil are moderate CYP 3A4 inhibitors
And here are a few clinical pearls about diltiazem and verapamil:
Diltiazem may get the patient to better BP goal than the other agents. But do not infuse a diltiazem drip for longer than 24 hours or faster than 15 mg/hr (because there is no data to support faster/longer infusion).
Verapamil causes more profound hypotension compared to diltiazem. It's also got a risk of gingival hyperplasia (yes, the same thingyou learned about with phenytoin).
2. Beta Blockers (BBs)
We tend to prefer short acting BBs (like esmolol or propranolol) in the acute setting. Like CCBs, BBs can be risky in heart failure patients. But the risk is lower for drugs (especially esmolol) that have a super short half-life.
Esmolol - In many facilities, it is reserved for ICU patients (it's used for aortic dissections and strokes). It's got an incredibly short half-life (9 minutes, to be exact). Some people remember this with the phrase: "It's small LOL."
Propranolol - Is a CYP 1A2 substrate. Also keep in mind that it's a non-selective BB. This means that it's got a wide array of side effects, but also that you can see it used for atypical indications such as migraine prophylaxis, stage fright, and anxiety.
3. Amiodarone
We'll discuss this in a moment...
4. Digoxin
We'll also discuss this in a moment...
So, we mentioned above that CCBs and BBs are risky for our heart failure patients. If they're out, what are our remaining options?
Either amiodarone or digoxin.
Digoxin
Purple Foxglove: The origin story for digoxin. (Image)
Digoxin has been around for centuries—the extract of the purple foxglove plant that was used to treat dropsy (an old name for edema that results from heart failure).
Unlike CCBs and BBs, Digoxin is a positive inotrope so it will not depress the EF. As you're probably aware, however, digoxin is far from perfect.
It works by enhancing the parasympathetic activity in the AV node.
This means it is good for slowing the heart rate at rest, but not as good during exercise (since sympathetic activity can override its effects).
It also has a narrow therapeutic window and one of its main toxicities is arrhythmia (plus some good old fashioned confusion, nausea/vomiting, and yellow-green halos in the vision).
Therapeutic drug monitoring is necessary if the patient is experiencing arrhythmia, since it is difficult to determine clinically if these patients have too high or too low of a digoxin level.
Oh, and there are tons of drug interactions with digoxin (it's a CYP3A4 and P-glycoprotein substrate).
Amiodarone
Speaking of drug interactions, amiodarone is notorious for these as well (it inhibits CYP3A4, CYP2D6, and P-glycoprotein).
Amiodarone is a Class III antiarrhythmic that, like digoxin, can be used in heart failure. It's quite a versatile drug--It can be used as both a rate controlling agent AND a rhythm controlling agent for Ventricular Tachycardia (VT), Pulseless VT (you'll see it in the ACLS Guidelines), and afib.
It can also be used as a pharmacological cardioversion agent. Say what you will about amiodarone, but the drug is versatile.
Amiodarone can cause a lot of fun side effects like pulmonary toxicity, Torsades de Pointes, phospholipidosis, corneal deposits, blueish/gray skin, and hypotension.
One of the biggest things to remember about amiodarone is that it has a long and variable half-life of 9-142 days (yes, you read that right).
Little known fact: This song was written about amiodarone (Image)
When I think of amiodarone, I think of the Journey song “Don’t Stop Believing” because amiodarone “Goes on and on and on and onnnn...”
Now you either want to take a study break to listen to Journey (don't worry, I linked it above) or slap me across the face for bringing this catchy song up.
Lucky for me, I am on the other side of a computer screen so you will have to resort to throwing a pencil/stapler/other thing across the room instead.
Now that you are either done listening to Journey or have cleared off your desk a bit (you’re welcome), let’s talk briefly about outpatient rate control in afib (remember, our discussion above was focused on treating afib in the acute setting).
Outpatient Rate Control Agents for Afib
Outpatients can be maintained on non-dihydropyridine calcium channel blockers (diltiazem, verapamil) or most beta blockers (atenolol, metoprolol) to effectively reduce rate chronically.
Notice that we're not using the same beta blockers as in the acute setting. When we're talking about outpatients, the short half-life of esmolol and propranolol are undesirable.
You can use a combination therapy (but not drugs from the same class) or add digoxin if needed to achieve adequate control.
Based on the RACE II trial, lenient control of heart rate at 110 beats/min or less was non-inferior to strict rate control of <80 beats/min (as long as left ventricular systolic function is preserved) in the prevention of major cardiovascular outcomes.
For highly symptomatic patients, a lower heart rate goal may be considered.
Again, we prefer rate control since most patients can be controlled with rate alone and anti-arrhythmic agents tend to be pro-arrhythmic (not to mention all the other nasty side effects/drug interactions).
So when would you use rhythm control?
Consider using chronic rhythm control in patients with tachycardia-induced myopathy or continuing rhythm control in a stable afib patient.
Some of our options for chronic rhythm control include:
Propafenone
Dofetilide
Dronedarone
Sotalol
And good ol’ amiodarone (“It goes on and on and on and onnnn.”)
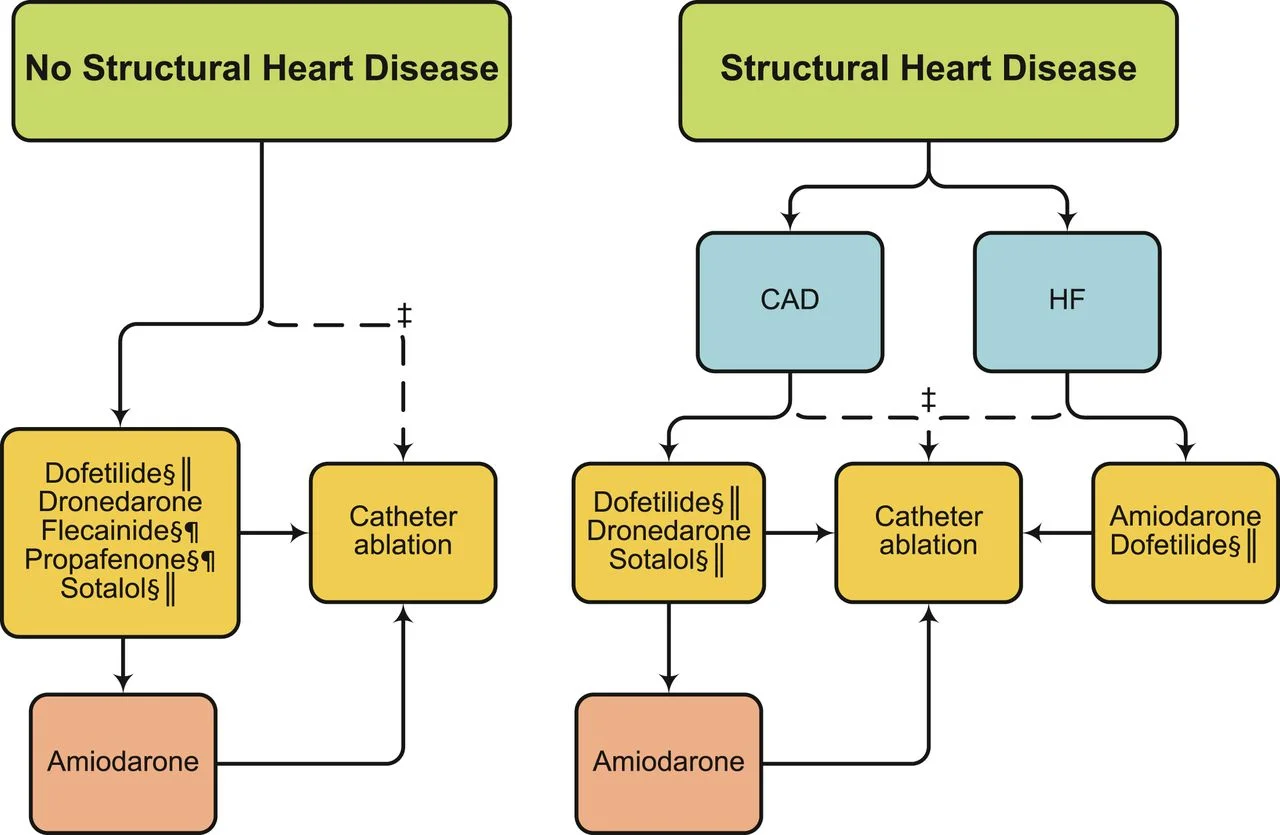
According to the AHA/ACC guidelines, here is a general schematic of which agent you can use considering structural heart issues.
Flecainide and Propafenone are considered Class IC antiarrhythmic agents.
Amiodarone, Dofetilide, and Dronederone and Sotalol are Class III antiarrhythmic agents.
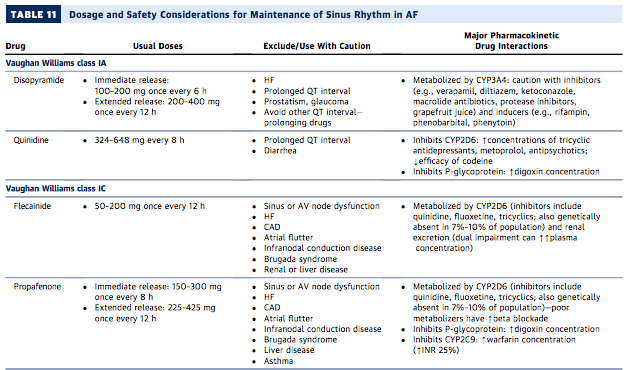
It is difficult to easily mention all of the issues with each drug individually, so I will refer you to this handy chart from the AHA/ACC/HRS Guidelines:
Once the patient is not acutely decompensating, our next important consideration is preventing strokes/clots. The major cause of morbidity from atrial fibrillation is from the increased risk of stroke.
Remember Jeremiah the Innocent and the tale of the triple clot man from earlier? If not, ctrl-F “mural,” in the atrial fibrillation pathophysiology section, and you will be reminded.
I won't delve into the nitty gritty of blood thinner (anticoagulation) treatment, since there is already a great tl;dr article on this – Anticoagulants: the Definitive Guide. They've also got a really handy anticoagulant cheat sheet that really makes the memorization process easy. You can get your copy of it here.
Just know that anticoagulation is extremely important for afib patients and cannot be ignored.
Once the patient is rate/rhythm controlled, anticoagulated, and maintained with persistent afib, we want to think about cardioversion (i.e. returning the patient to NSR).
Our options are either direct current (electrical) cardioversion or pharmacological cardioversion to return the patient to NSR.
For both options, you must anticoagulate for at least 3 weeks prior to cardioversion (regardless of CHA2DS2Vasc Score ) and at least 4 weeks after cardioversion.
You can shorten the duration of anticoagulation with a scan of the heart/vessels (TEE – transesophageal echocardiogram). As long as no thrombus is seen on the TEE, you don't have to do the full 3 weeks (but you DO need to make sure the patient is fully anticoagulated prior to cardioversion).
(Image)
Direct Current Cardioversion (DCC) is probably the best option for conversion to NSR if the patient can be sedated (since getting shocked by electricity is quite uncomfortable.)
DCC has a higher success rate and shorter hospital stay overall compared to pharmacological conversion.
For pharmacological conversion, we can use our antiarrhythmic agents (the ones we mentioned earlier for rhythm control). For cardioversion, we use them at higher doses for a shorter periods of time in an attempt to convert the rhythm permanently.
There are a few options for pharmacological conversion, including:
Propafenone
Flecainide
Dofetilide
Ibutilide
And amiodarone (“It goes on and on and on and onnnn.”)
Dofetilide/ibutilide must be done in-hospital, whereas propafenone or flecainide (plus rate control) can be done on an outpatient basis once the patient is no longer in acute danger.
Propafenone or flecainide have been shown to be the most effective at converting to NSR; but amiodarone has a long history (and is pretty safe with short term therapy).
If you've tried several times to cardiovert with pharmacologic/DCC options and the patient is still symptomatic, it's time to consider catheter ablation.
Catheter ablation is a surgical procedure in which the electrical signals causing the arrhythmia are physically zapped, damaging those sections of the heart to allow the SA node to take control again.
The AHA/ACC guidelines suggest that Catheter ablation can be considered after > 12 months of persistent afib refractory to at least 1 antiarrhythmic agent.
Atrial Fibrillation Treatment Summary
Let's recap some lessons we’ve learned about atrial fibrillation treatment:
DCC may be necessary in patients with atrial fibrillation with rapid ventricular rate who also appear extremely ill (i.e., acute decompensated heart failure, hypoxic.)
Controlling rate is EASIER than controlling the rhythm, so rate control is usually the way to go.
The major cause of morbidity from atrial fibrillation is from strokes, so anticoagulation is key.
DCC and pharmacological cardioversion to NSR can be considered after at least 3 weeks of successful anticoagulation (and you'll need 4 more weeks of anticoagulation after successful cardioversion).
Catheter ablation is used after rate and rhythm control have failed to convert the patient to NSR
Dosing Information Diltiazem, Digoxin, and Amiodarone
Oh, did you think we were finished? Not even close. For your future reference, here are some standard loading doses for diltiazem, digoxin, and amiodarone.
How do I slow RVR using diltiazem?
Diltiazem is probably our "go to" drug for patients that don't have heart failure.
After confirming the patient is hemodynamically stable and does not have a history of systolic HF (HFrEF) with an EF < 40%.
Obtain peripheral IV access.
Give a slow IV bolus of diltiazem over 2 minutes, 0.25 mg/kg (actual body weight) which generally translates to 15-25 mg for most adults
If unsuccessful, you may repeat at 0.35 mg/kg (again over 2 minutes).
Immediately follow this with an initial infusion rate of 10 mg/hour; rate may be increased in 5 mg/hour increments up to 15 mg/hour as needed; some patients may respond to an initial rate of 5 mg/hour.
You should likely convert to PO within 24 hours; the conversion is: Oral dose (mg daily) is approximately equal to [rate (mg/hour) x 3 + 3] x 10. The long acting formulations come in 120mg, 180mg, 240mg and 360mg. We try to round down if the calculated dose is in the middle of the range, but just use common sense.
How do I slow RVR using digoxin?
We generally use digoxin for patients who have a low EF or have symptoms at rest.
To emergently or urgently digitalize (I don’t think it is a real word, but I like it) a patient:
Calculate the total loading dose of 8-12 mcg/kg of LEAN body mass.
Then give ~1/2 of the dose IV over 5 minutes.
Give the remaining two quarters of the loading dose 4-12 hours after each dose.
For example, a 100kg man who has a lean body mass of about 75 kg (we use EBMConsult.com).
75 kg x 10 mcg/kg = 750 mcg = total loading dose (it comes in a 500 mcg/vial or 250 mcg/1 mL)
375 mcg IV over 5 minutes ----------> 1mL/250 mcg x 375 mcg/dose = 1.5 mL
4-12 hours later 184.5 mcg which will translate into 0.75 mL
4-12 hours later repeat the 184.5 mcg or 0.75 mL
Wait 24 hours before beginning maintenance dosing, if over age is 65 or if the patient is frail, use 125 mcg daily. If RESTING heart rate is < 60 beats/min, you may need to dose every other day. If RESTING heart rate is >80, consider 250 mcg daily.
How do I slow RVR using amiodarone?
Amiodarone can be used for both rate and rhythm control in AF. That is, it may slow the rate and/or convert afib to NSR.
To slow afib with RVR, most clinicians use a 300 mg IV loading dose given over 30 min followed by an infusion.
In a short paper, Tuseth, Jaatun and Dickstein looked at infusion rates of 50 mg/hr and 100 mg/hr for the next 24 hours. The high dose group achieved conversion to NSR much sooner than the lower dose, but this divergence did not occur for 10 hours and both strategies were effective for rate control (so in a nutshell, you can use either).
So that's:
300 mg IV over 30 minutes
Followed by 50 - 100 mg/hr infusion
If you're NOT dealing with RVR, you may also use the following scheme (Note: this is also used by some clinicians for patients with RVR):
Load 150 mg over 10 min
Followed by 1 mg/min for 6 hours
Then 0.5 mg/min for 18 hours.
If you're unable to convert to PO before 24 hours, consider decreasing the IV dose to 0.25 mg/min.
No matter which strategy you follow with amiodarone, the goal is to transition to PO within 24 hours. You're typically looking at 400–600 mg daily in divided
doses for 2–4 weeks. Then you'd transition to a maintenance dose of 100 - 200 mg daily.
As a final note, for all of these drugs (diltiazem, digoxin, and amiodarone), if patients become unstable anytime during the load or the infusion, electrical cardioversion is the standard of care.