How to Prevent (and Treat) Febrile Neutropenia
Editor’s Note: Boramey Pom is a P4 student at the University of Texas College of Pharmacy at Austin. He also has had the unfortunate experience of spending 6 weeks with me on an APPE rotation. Please keep Boramey in your thoughts and prayers. One of Boramey’s assignments on rotation was to help me write this post on Febrile Neutropenia, and he did a fantastic job. You’ll learn a lot from reading this. After graduation, Boramey plans to pursue a residency. In the very limited time he has outside of pharmacy, he enjoys traveling, playing basketball, learning jiu jitsu, and bouldering.
BTW - We compiled ALL of our Oncology Supportive Care posts into one handy, downloadable (and printer-friendly) PDF. You can get your copy of it here.
Febrile Neutropenia Pathophysiology
When we think of the side effects of chemotherapy, the first visuals that come to mind are usually hair loss and vomiting. The topic for today is a bit more discreet than projectile vomit, but potentially more serious.
Febrile neutropenia (also known as neutropenic fever or neutropenic sepsis) is one of a handful of oncologic emergencies. It’s in the same notorious group as TLS, hypercalcemia, and spinal cord compression. But unlike the other oncologic emergencies, neutropenic fever is relatively asymptomatic. In fact, fever is often the only symptom present. That doesn’t sound so bad, right? Your 3-year-old nephew gets fevers all the time, and you’re not rushing him to the ER. What gives?
Let’s take a step back. Very broadly speaking, cancer therapy is broken down into 3 main categories:
Surgery
Radiation
Systemic Therapy
It’s mostly with systemic therapy that we are concerned about neutropenic fever.
Systemic therapy is broken down into 4 main branches:
Cytotoxic Chemotherapy
Targeted Therapy
Immunotherapy
Hormonal Therapy
Rapidly dividing cells are also error-prone…
And for the purposes of febrile neutropenia, we’re primarily concerned with cytotoxic chemotherapy. Basically, it’s an “atomic bomb” approach. Cytotoxic therapy works by killing cells that are actively undergoing cell division. Cancer is a disease that is pretty much defined as rapid, uncontrolled cell growth. So, great! Cytotoxic chemotherapy kills rapidly growing cells. Cancer is made up of rapidly growing cells. What’s not to love? Unfortunately, there are plenty of cells in your body that rapidly grow under normal conditions. Your hair, the lining of your GI tract, and your bone marrow.
So, in the pursuit of killing your cancerous cells, some systemic chemotherapies wipe out your white blood cells (which are made in your bone marrow). Neutrophils are an important type of white blood cell that helps you fight off bacterial pathogens. And many neutrophils are lost as collateral damage when cytotoxic chemotherapy is given.
Low neutrophils make it really hard for your body to fight off infections (especially bacterial ones). In fact, a fever is about the only way your body can fight infection without its white blood cell army.
And that’s the crux of neutropenic fever. It’s an oncologic emergency because your body has no other means to fight off infections. Patients undergoing cytotoxic chemotherapy that develop fevers are treated very seriously. These patients are often treated emergently, as if we were treating sepsis…because there’s a good chance the patient is septic.
Febrile Neutropenia Diagnosis
So, what defines febrile neutropenia? As you can probably surmise, you need to meet 2 conditions — fever and low neutrophils. But again, these patients can be relatively asymptomatic (other than the fever), so you almost have to be looking for febrile neutropenia in order to diagnose it. In terms of “official” cut-offs, this is what we're looking for:
Temperature: A single temperature of ≥ 38.3°C (101°F) or a temp of 38.0°C (100.4°F) sustained for an hour
Absolute Neutrophil Count (ANC): ANC of ≤ 500 cells/uL OR an ANC of 1000 cells/uL with an anticipated decrease to 500 cells/uL over the next 48 hours
As an FYI, in clinical practice (at least in the US), we often normalize the ANC into cells/mL (instead of uL). So, instead of saying, “This patient has an ANC of 1500,” we might say, “This patient has an ANC of 1.5.”
The diagnostic criteria for febrile neutropenia listed above can seem a bit ambiguous. It sorta leaves a bit of room for interpretation, doesn’t it? I mean, when would one “anticipate” a drop in the ANC to 500? We’ll get to that in a bit. First, let’s talk about how to calculate the Absolute Neutrophil Count.
Many Electronic Medical Records will actually provide you with the ANC. So you won’t have any math problems to solve. But that’s not always the case. Some outside labs do NOT directly provide you with the ANC. Sometimes (for example, on the NAPLEX), you may have to calculate it. But don’t worry, it’s pretty simple. There are two ways you can do it (and you should know how to do both because both scenarios can show up when you’re practicing as a pharmacist).
In the first scenario, you won’t have the absolute neutrophil count, but you will have the neutrophil percentage. The neutrophil percentage is (spoiler alert) the percentage of your white blood cells that are neutrophils. You simply have to multiply the neutrophil percentage by the total number of white blood cells to get the ANC. So, if your WBC count is 2.8, and your neutrophil % is 25%, you’d do the following:
WBC x Neutrophil% = ANC
2.8 x 0.25 = 0.7 cells/mL
Again, in human words, you would say that your patient has an ANC of 0.7 (or an ANC of 700 depending on the units you’re using).
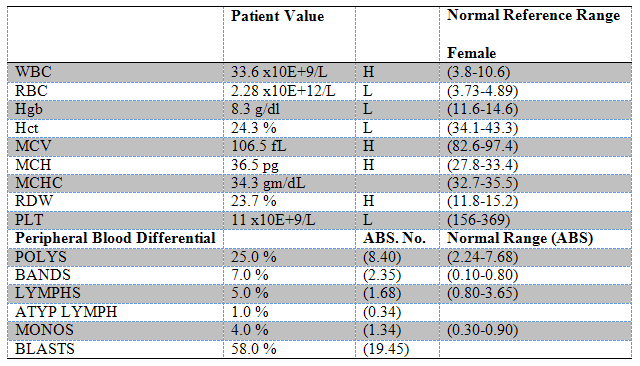
In the second scenario, you have the total WBC count, but you don’t have the neutrophil percentage. Instead, you’ll have the full differential on the CBC. It’ll look something like this:
We’ll ignore the leukemic blast crisis that this patient has for the purposes of this demonstration. (Image)
Now, let’s take a brief detour and look at the maturation process of a neutrophil:
*Sniff* Our little baby neutrophil is growing up so fast! (Image)
Notice how we call mature neutrophils “segmented” neutrophils? Segmented neutrophils are also known as “Segs” or “Polys.” They’ve earned these nicknames because of the segmented or polymorphic look of the nucleus. Next, notice that before we have a mature segmented neutrophil, we have a Band cell (because the nucleus looks like a band). Band cells (aka “Bands”) are less mature neutrophils. They still have an important role in our immune system, but they aren’t fully differentiated like a Seg.
Now, look again at our CBC with differential above. Do you see how we have the percentage of Polys (again, this could also be called Segs), and the percentage of Bands? If you add those two up, you’ll have your percentage of neutrophils. So, to figure out the ANC in this patient, you’d multiply the WBC count by the Polys% + the Bands%. It looks like this:
ANC = WBCs x (Polys% + Bands%)
ANC = 33.6 x (0.25 + 0.07)
ANC = 10.75
Pretty easy, right? Now you’ll be able to calculate the Absolute Neutrophil Count no matter what information you’re given.
Neutropenic Fever Risk Stratification
An important part of diagnosing febrile neutropenia is to figure out the risk that your patient will have serious complications. This is an important step because it steers the direction that your treatment plan will go. We’ve got several validated scoring systems that help us figure out if we can treat our neutropenic patient as an outpatient or if we need to admit them for IV antibiotics. The two main scoring systems in use are:
The Multinational Association for Care in Cancer (MASCC) Score
The Clinical Index of Stable Febrile Neutropenia (CISNE) Score
These systems are pretty similar, but there are some subtle differences. Both of them start with the assumption that your patient already has febrile neutropenia.
Comparing the criteria of each score will help us get a handle on how to make the best clinical decision for our patients. The MASCC Score has been around longer and seems to be a bit more popular in the US, so we’ll start there.
Contrary to most risk factor scales in the healthcare field, the higher your MASCC Score, the lower your risk of complications from febrile neutropenia. The highest MASCC Score one could get is a 26. And in terms of risk stratification, patients are divided into two groups.
A score of ≥ 21 = low risk for serious complications
A score of < 21 = high risk for serious complications
The takeaway from this is that, if your patient has a high enough MASCC Score (low risk for complications), you can likely treat their febrile neutropenia as an outpatient (with very close monitoring and follow-up, obviously). If they have a low MASCC Score, they’ll do better if you admit them. You can also see that there seems to be a lower risk of neutropenic fever complications with solid tumors compared to hematologic malignancies.
To contrast, let’s now look at the CISNE Score. This is the newer kid on the block, but it’s starting to gain some traction.
Its proponents point to the fact that it is more specific and objective than the MASCC Score. Instead of subjective scores like “mild” or “moderate” symptoms on the MASCC, the CISNE uses the objective ECOG Performance Status. It also includes your monocyte (aka macrophage) count in the scoring. If your patient’s monocyte count is low in addition to them being neutropenic, that’s a negative prognostic marker. Also, we’ve seen from practice that if a patient’s monocyte number is increasing that the neutrophils are likely to follow soon afterward.
The CISNE also gives you 3 levels of risk:
Low Risk (score = 0)
Intermediate Risk (score = 1 to 2)
High Risk (score ≥ 3)
So you might say the CISNE is a bit more nuanced than the MASCC. But again, we’ve got decades of use with MASCC, and at the end of the day, no scoring system is perfect. Both the MASCC and the CISNE can help you make the best clinical decision for your patient.
We could muddy the waters a little further and tell you that the IDSA has a joint publication with ASCO with their position on neutropenic fever. And NCCN has its own guidelines for the prevention and treatment of cancer-related infection. But there is a lot of overlap with what we’ve already discussed, and we’ll dive deeper into prevention/treatment in a bit.
One last diagnostic bit we should discuss is the difference between neutropenia and profound neutropenia. Profound neutropenia is when the ANC is ≤ 100 cells/uL…and it’s particularly worrisome if the counts are expected to stay that low for 7 days or more. In practice, these patients tend to be those undergoing stem cell transplants, and the therapeutic management doesn’t differ from the run of the mill “neutropenia” (ANC ≤ 500 cells/uL). But the guidelines mention the distinction, so it’s a good idea to keep it in mind.
Combining everything we’ve covered so far, we might make a general (read: oversimplified) chart that looks like this:
Febrile Neutropenia Treatment
Let’s review what our 2 criteria for neutropenic fever diagnosis were:
Temperature: A single temperature of ≥ 38.3°C (101°F) or a temp of 38.0°C (100.4°F) sustained for an hour
Absolute Neutrophil Count (ANC): ANC of ≤ 500 cells/uL OR an ANC of 1000 cells/uL and anticipating a decrease to 500 cells/uL over the next 48 hours
How do you “anticipate” that your patient’s ANC will drop to below 500 within 48 hours? There isn’t a hard and fast answer, but we can make some general predictions. As we said above, it’s mostly systemic cytotoxic therapy that affects the neutrophils. So if your patient is receiving cytotoxic drugs (anthracyclines, taxanes, alkylating agents, etc), then that’s the first step to making an educated guess. The second step involves knowing the nadir of the patient’s chemotherapy regimen.
The nadir is the point in time in which you’d expect to see the maximum amount of bone marrow suppression from a given chemo regimen. It’s when you would expect your patient’s ANC to reach its low point. It’s like a trough - but for your blood cells. Physiologically, it tends to occur at the same time a patient is most likely to experience mucositis (which makes sense, because mucositis is also due to cellular toxicity from the chemotherapy). For most chemo regimens, the nadir occurs in about 9 or 10 days (but it can range from 7 - 14 days depending on the regimen). This gives us a bit of predictive capability when anticipating neutropenia.
Let’s say that a patient receives chemotherapy on Day 1, and their ANC is 600 on Day 7. Knowing that the nadir is usually seen around Day 9 or Day 10, we could reasonably expect that this patient will be neutropenic in 48 hours, and we can act accordingly.
It’s worth noting a few things here. With mucositis occurring at the same time point as the nadir, the patient is at serious risk of having mouth/gut flora translocate into the bloodstream. It’s best to avoid poking and prodding any part of the GI tract. That means you should avoid taking rectal temperatures, giving suppositories, or doing any kind of rectal examination. Even seemingly minor injuries can become sepsis in a hurry.
The second thing to note is that although it’s OK to give acetaminophen as needed for fever, you should avoid giving it scheduled around the clock. This includes opiate/acetaminophen combinations such as Norco or pretty much any over the counter cold/sinus medication. Suppressing the fever may make the patient feel better, but it will also make it more difficult to monitor and manage the patient’s febrile neutropenia.
With that out of the way, let’s move on to treatment. Treatment of neutropenic fever is stratified based on whether the patient is at high risk or low risk (based on the MASCC or CISNE Score). As we said before, higher-risk patients should be admitted and treated in a hospital with IV antibiotics. With lower-risk patients, you can at least consider outpatient therapy and oral antibiotics. That said, MASCC Score be damned, it is still a clinical decision that has to be made for each and every patient. The last thing you want to do is to under treat a patient and have something go south.
So, we’ve got a confirmed diagnosis of febrile neutropenia. Our patient is at risk for bacterial infections. We need antibiotics (and we need them ASAP). But which antibiotics?
If possible, the first step is to get cultures. We’ll do this even before giving an antibiotic. It will help us identify the pathogen(s) causing the infection, which will let us streamline our therapy later. Cultures are especially critical for your high-risk patients (even more so if they’re anywhere on the SIRS/Sepsis spectrum). After cultures are drawn, we need an empiric antibiotic regimen.
When it comes to designing empiric antibiotic regimens, you have to take a few things into account. The first question to ask is what pathogens are you concerned about? As we just said, because of mucositis we’ve got a risk of gut flora translocating to the bloodstream and causing sepsis. With our mucosal barriers compromised, our empiric regimen needs to cover common gut flora. E. coli, Klebsiella, and Enterococcus are common offenders here. But these patients are also immunosuppressed, so we need to bring an extra level of caution to the table. That means stepping it up to include Pseudomonas coverage as well.
Generally speaking, we also need to consider patient-specific factors to see what (if anything else) we need to cover. If the patient presents with cough or dyspnea, we’d have to consider respiratory infections. If they’re experiencing painful urination, we need to cover UTIs. If they have an active case of oral thrush, we’ve gotta think about Candidiasis. If they have any CNS symptoms (especially if you see the term “encephalitis” in the mix), then you’ll be adding IV acyclovir to the mix.
Your instincts may tell you that we should cover for MRSA. There’s a tendency to want to throw the kitchen sink at a patient with neutropenic fever, and a little bit of vancomycin never hurt anyone, right? Not exactly. There are specific patients where we’d include coverage for MRSA, but it’s not necessary for everyone. Obviously, if the patient has a history of MRSA infection, we should make sure we’re covering that. Additionally, if a patient has a chemotherapy port (such as a portacath), it could be a source of infection. And infected chemo ports grow MRSA frequently enough that we’ll usually empirically treat for MRSA.
If you’ve seen our Antibiotic Cheat Sheet (get it for free by joining our email list!), you’ll see that there aren’t that many treatment options for Pseudomonas and MRSA. You’ll do yourself a favor if you can commit them to memory. You can also check out our quick and dirty guide to antibiotics to learn more.
So, we’ll cover the gut flora, including Pseudomonas. Plus or minus MRSA coverage and other pathogens as determined by patient-specific factors. Obviously, we’ll also account for patient allergies and renal/hepatic function. Where does that leave us?
If our patient is Low Risk according to the MASCC or CISNE Score, we’d likely hit them with a PO combination of amoxicillin/clavulanate (Augmentin) PLUS ciprofloxacin. That will cover most of the GI baddies, while still giving us some (modest) Pseudomonal coverage.
If our patient is High Risk, cefepime is probably the best choice for most patients. Adding the anaerobic coverage of piperacillin-tazobactam (Zosyn) or the “kill them all” coverage of meropenem is probably overkill unless there is a specific history of resistance in the patient. For example, if the patient has a history of infection with ESBL-producing organisms, carbapenems should be your default choice. If we’ve got any suspicion for MRSA, we’ll add vancomycin.
This is you waving “buh-bye” to vancomycin after the blood cultures grew out gram negative rods
Obviously, that’s a bit of an oversimplification. But we’ll be here all day if we try to dissect every potential scenario. In a pinch, the above rules will cover you for most patients. Don’t forget to streamline your antibiotic regimen once you’ve got a pathogen identified.
Let’s say it’s been a few days, and your patient isn’t getting much better. They’ve still got a fever and they don’t seem to be clinically responding. At this point in time, you’ll think about expanding your coverage and potentially adding antifungals to the mix.
Usually, if you’ve reached this fork in the road, you need to empirically cover for molds (like Aspergillus). Your drug of choice here will likely be voriconazole, but you’ve got a few options (check out our Beginner’s Guide to Antifungals to get your bearings).
The duration of therapy will ultimately depend on what your patient was infected with. But a good rule of thumb is to treat at least until your patient is afebrile and their ANC is above 500.
Febrile Neutropenia Prophylaxis
Phew! This has been a marathon, but we’re almost there.
Do we ever try to prevent all the nasty infections from the previous section? The short answer is “Yes, but not as often as you might think.” For most solid tumors (remember, these are usually low risk for neutropenic fever), we don’t provide any routine prophylaxis. You might consider giving someone who routinely suffers from cold sores prophylactic acyclovir or valacyclovir, but that’s about it.
Things get a little dicier when we talk about our hematologic cancers. This includes leukemia, lymphoma, myelodysplastic syndrome, and multiple myeloma. Now we’ll start pumping up our preventative muscles, but again, it varies by disease state. In general, acute leukemias (ALL and AML) pose the greatest risk and the greatest need for prophylaxis. For example, AML patients are extremely high risk, and you’ll often give preventative antibiotics, antivirals, and antifungals. Notably, AML patients are particularly at risk for mold infections, so it’s best to use posaconazole (or something else that covers molds) when they’re at risk of being neutropenic. Also, the steroids that are a mainstay of ALL treatment often make PJP prophylaxis a must, so Bactrim is a frequent flyer in these patients.
Check the most recent version of the NCCN Guidelines for the Prevention and Treatment of Infections to get the most up to date recommendations.
So that covers the “febrile” part of febrile neutropenia…but what about the “neutropenia” part? We’ve got options there too.
Granulocyte-Colony Stimulating Factor (G-CSF) can be used to help the body produce new neutrophils. We can use these to prevent or treat neutropenia. How do you choose a G-CSF? Honestly, it boils down to convenience and formulary preference.
Here's a quick break down:
filgrastim [Neupogen] - 5 - 10 mcg/kg/day daily SubQ or IV
filgrastim-sndz [Zarxio] - biosimilar for Neupogen. The same dosing applies.
filgrastim-ayow [Releuko] - biosimilar for Neupogen. The same dosing applies.
tbo-filgrastim [Granix] - It's kind of like a biosimilar for Neupogen...but technically it obtained it's own FDA approval before the biosimilar program existed (so you cannot call it a biosimilar). It is not FDA approved for as many indications as Neupogen or Zarxio, but it is used in practice for all of the same things. For what it's worth, CMS has assigned the same J-code to Neupogen, Zarxio, and Granix. This basically means that CMS considers them interchangeable. The dosing is the same among all 3 agents.
pegfilgrastim [Neulasta], [Neulasta Onpro] - 6 mg SubQ once per cycle of myelosuppressive chemotherapy.
pegfilgrastim-jmdb [Fulphila] - biosimilar for Neulasta. The same dosing applies.
pegfilgrastim-cbqv [Udenyca] - biosimilar for Neulasta. The same dosing applies.
pegfilgrastim-bvez [Ziextenzo] - biosimilar for Neulasta. The same dosing applies.
pegfilgrastim-apgf [Nyvepria] - biosimilar for Neulasta. The same dosing applies.
pegfilgrastim-fpgk [Stimufend] - biosimilar for Neulasta. The same dosing applies.
sargramostim [Leukine] - 250 mcg/m2/day daily SubQ or IV. Leukine is actually a GM-CSF (it stimulates both granulocytes and macrophages). It's primarily used for stem cell/bone marrow transplants, although it also has an indication for AML.
Holy cow, that’s a lot of biosimilars. If the concept of biosimilars confuses you, you can get our quick and dirty summary here.
G-CSFs (and GM-CSFs) are pretty well-tolerated, but here are a few quick clinical pearls...
Accurate depiction of G-CSF for many patients…
First up, pegfilgrastim is NEVER for the treatment of neutropenia. It’s only for prevention only. All formulations of pegfilgrastim (except the Neulasta Onpro) are given 24 - 72 hours after chemotherapy. Why? If we give them before or during chemotherapy, all of the neutrophils they stimulate are going to be killed by the chemo. The Neulasta Onpro is an "on-body injector" that is placed on the patient on the same day of chemotherapy. It auto-injects itself 27 hours later. This saves the patient from returning to the infusion center a day or two later for Neulasta (which is great if transportation is an issue). It’s worth noting, however, that the patient cannot shower while wearing Onpro.
You can also use filgrastim for the prevention of neutropenia. Its daily dosing makes it cumbersome for outpatients, but its lower cost makes it the go-to for inpatients. If you are treating active neutropenia, filgrastim is your only option.
If G-CSFs are so great, why don’t we give them to every patient that receives cytotoxic chemotherapy? Simply put, G-CSF is not benign, and not every chemo regimen is myelosuppressive. Why give an unnecessary drug if the patient isn’t likely to develop neutropenia?
How do you know when you should give prophylactic G-CSF? There are two different categories here. In some cases, a patient will only develop neutropenia after several cycles of chemo. In this case, we might add on G-CSF to prevent neutropenia from future cycles. This is called secondary prophylaxis. But some regimens cause so much myelosuppression that we use G-CSF as primary prophylaxis. This means that we don’t wait until the patient develops neutropenia, we give G-CSF starting with the very first cycle.
Once again, NCCN has a guideline of Hematopoietic Growth Factors to help you determine when it’s necessary to use primary prophylaxis with G-CSF. Historically speaking, if a chemotherapy drug (or regimen) has a risk of causing febrile neutropenia in at least 20% of patients, then it is appropriate to use primary prophylaxis.
One of the annoying side effects of G-CSFs is bone pain (which makes sense given their mechanism). Somewhat strangely, we can use antihistamines (typically loratadine) to help manage this pain. The worst "really bad thing" to watch out for is splenic rupture. G-CSFs (and GM-CSFs) can clog up your spleen. A ruptured spleen can be fatal, which is usually looked at as a negative adverse effect. There's also a small risk of anaphylaxis and other injection site reactions.
Neutropenic Fever - tl;dr
We’ve covered a lot of ground in this post. Let’s summarize with a few bullet points:
Cytotoxic chemotherapy can cause febrile neutropenia, which is an oncologic emergency.
These patients are particularly at risk for bacterial infections and must be treated with antibiotics.
In some higher-risk patients, we can try to prevent febrile neutropenia by giving prophylactic anti-infectives and/or G-CSF.
Never take Brandon’s APPE rotation…it’s not worth it.