An Introduction to Hematopoietic Stem Cell Transplant (HSCT)
Steph’s Note: This week, we’re doing yet another topic near and dear to my heart. No, I’m not an oncology pharmacist, but I am the daughter of a (matched-unrelated donor) allogeneic stem cell transplant recipient. So I assure you there are a LOT of principles that even non-oncology folks can take away and apply in their own practices.
Even though I feel strongly about this topic, like I said, I’m (soooo) not an oncology pharmacist. (I can’t - and really really shouldn’t - be the one to teach you this complicated topic.) This tall order falls to our oncology pharmacist extraordinaire, Olivia White! If you missed her hematologic malignancy primer earlier this year, I highly recommend checking that out in tandem with this post. Break it down for us, Olivia!
Now that we’ve had the opportunity to review the most common hematologic malignancies, it seemed like the next practical step for oncology on tl;dr is to review stem cell transplantation. This tends to be the ‘end goal’ for many patients (if they are able to tolerate this procedure) due to this being a potentially curative option for specific hematologic diseases.
On a related note, we’ll also briefly discuss chimeric antigen receptor T-cell therapy (CAR-T) due to these two strategies being somewhat comparable and them having overlapping indications for similar diseases.
What is BMT versus HSCT?
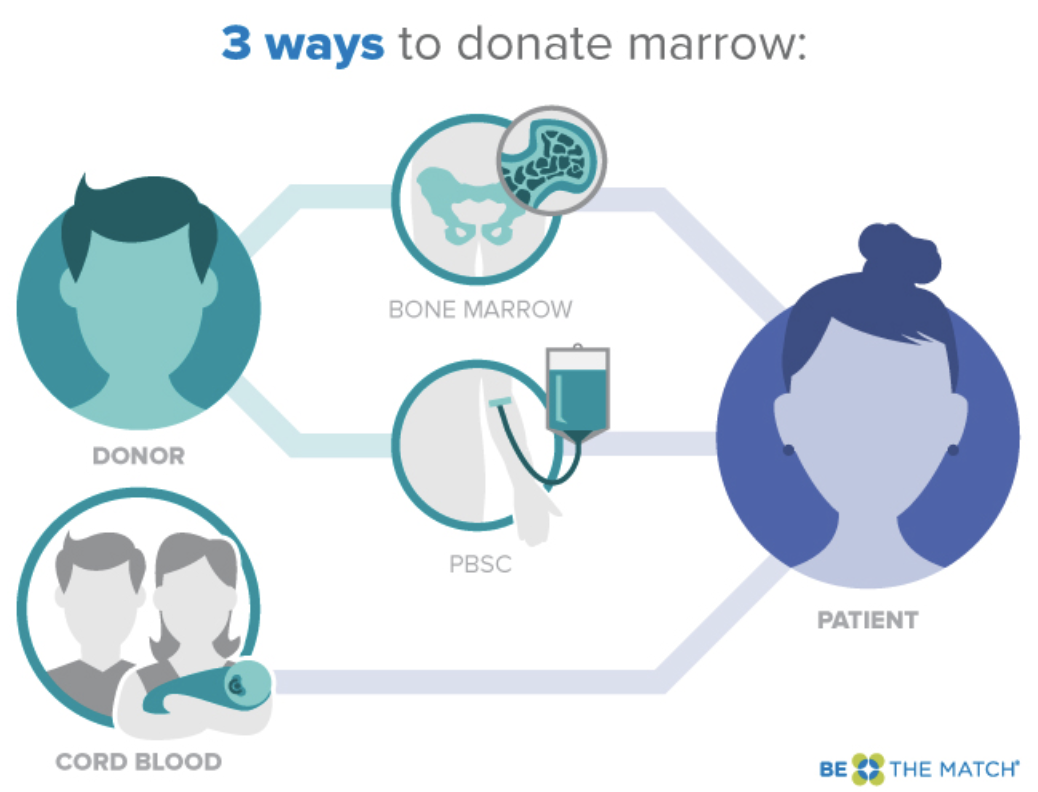
When comparing these two terms, it is critical that we clarify that these are not exactly the same thing; however, they are very similar. Bone marrow transplant (BMT) and hematopoietic stem cell transplant (HSCT) are two ways of collecting hematopoietic progenitor cells.
(Image)
When thinking about cell lineage, the initial cell is the hematopoietic stem cell, which is able to develop through its lineage into a wide variety of mature and functioning cells. BMT refers to the collection of these stem cells directly from the bone marrow. While this is the more ‘classic’ collection option, it is far less popular at this time in comparison to HSCT, which involves peripheral collection of stem cells from the bloodstream following cell mobilization.
We’ll be going over the different sources of stem cells later, but it’s important to note that the majority of stem cell donations are no longer collected from the bone marrow. This is due to the bone marrow donation process being much more laborious - and notoriously more painful - than current peripheral donation strategies.
The reason we are able to support this less invasive and less intensive process is due to this 2012 study. This was a phase 3, multicenter, randomized trial that compared peripheral blood stem cell collection to bone marrow collection. There was no significant survival difference between groups. However, it is important to note that the patients who received transplants from peripheral donations did experience more chronic graft versus host disease (GVHD).
More on GVHD later.
Nonetheless, this study has allowed us to use peripheral donation strategies in almost all of our donor populations!
Charlie’s an organ donor. Be like Charlie. But less grumpy. (Image)
PUBLIC SERVICE ANNOUNCEMENT!!!
We can’t have a HSCT post here without mentioning Be the Match. This is the national registry of bone marrow donors. (Shameless plug - the link to sign up is here. In case you didn’t click the hyperlink 3 seconds ago, here it is again. Seriously, like Nike says, just do it). I have had multiple friends that have had opportunities to donate - and Steph’s dad is the recipient of a kind, anonymous match! Take a couple minutes, and you just may save a life.
I mean, c’mon, it’s even easier than being an organ donor. It’s not like you permanently lose your stem cells if you donate!
PSA over…for now.
Learning the Lingo for HSCT
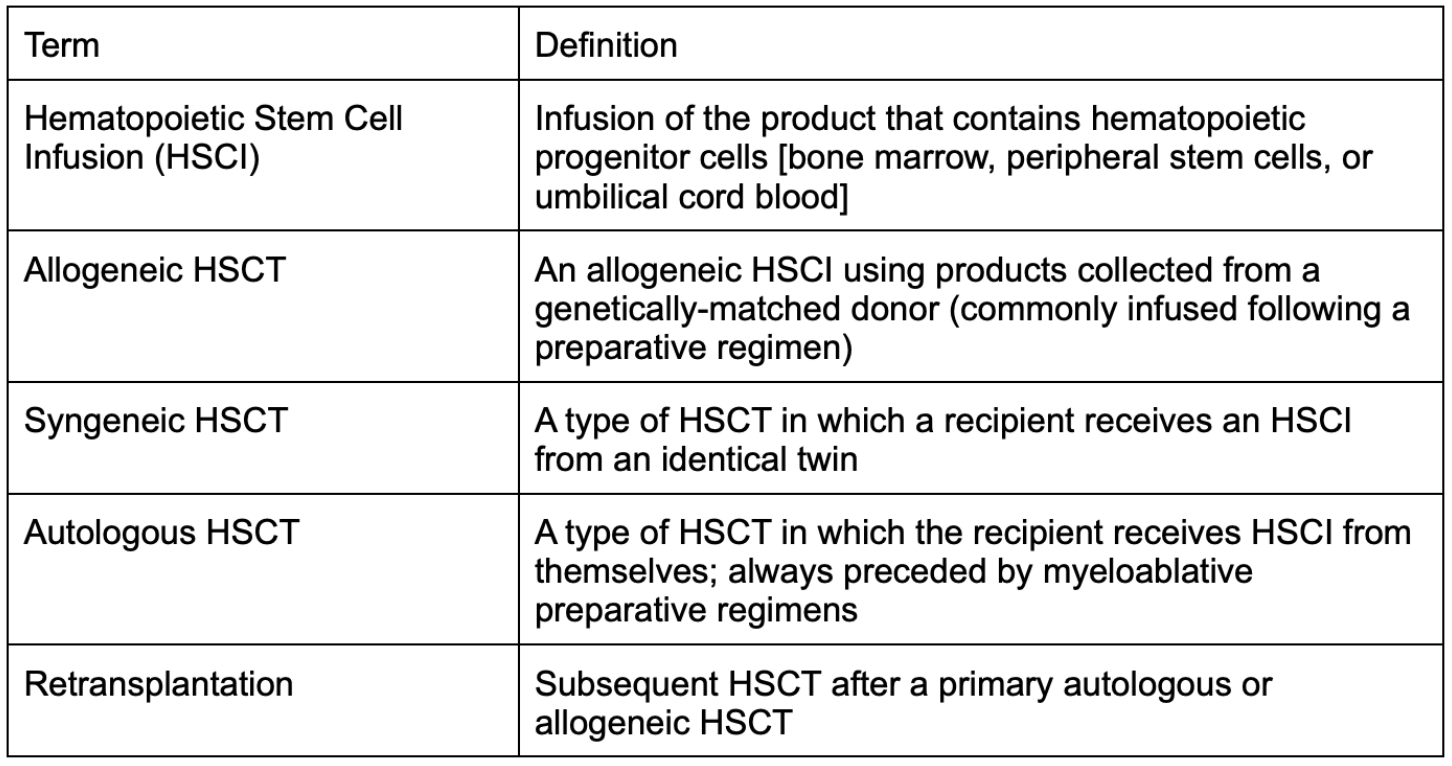
Before doing a deep dive into the mechanism that drives HSCT and the ins and outs of complications, regimens, etc., it’s key that we go over the common terminology used in the transplant world. (This is the primary resource I used for this section. I’ll hit the highlights, but they notably have a wonderful table for your reference, if you feel like you need even more of a refresher.)
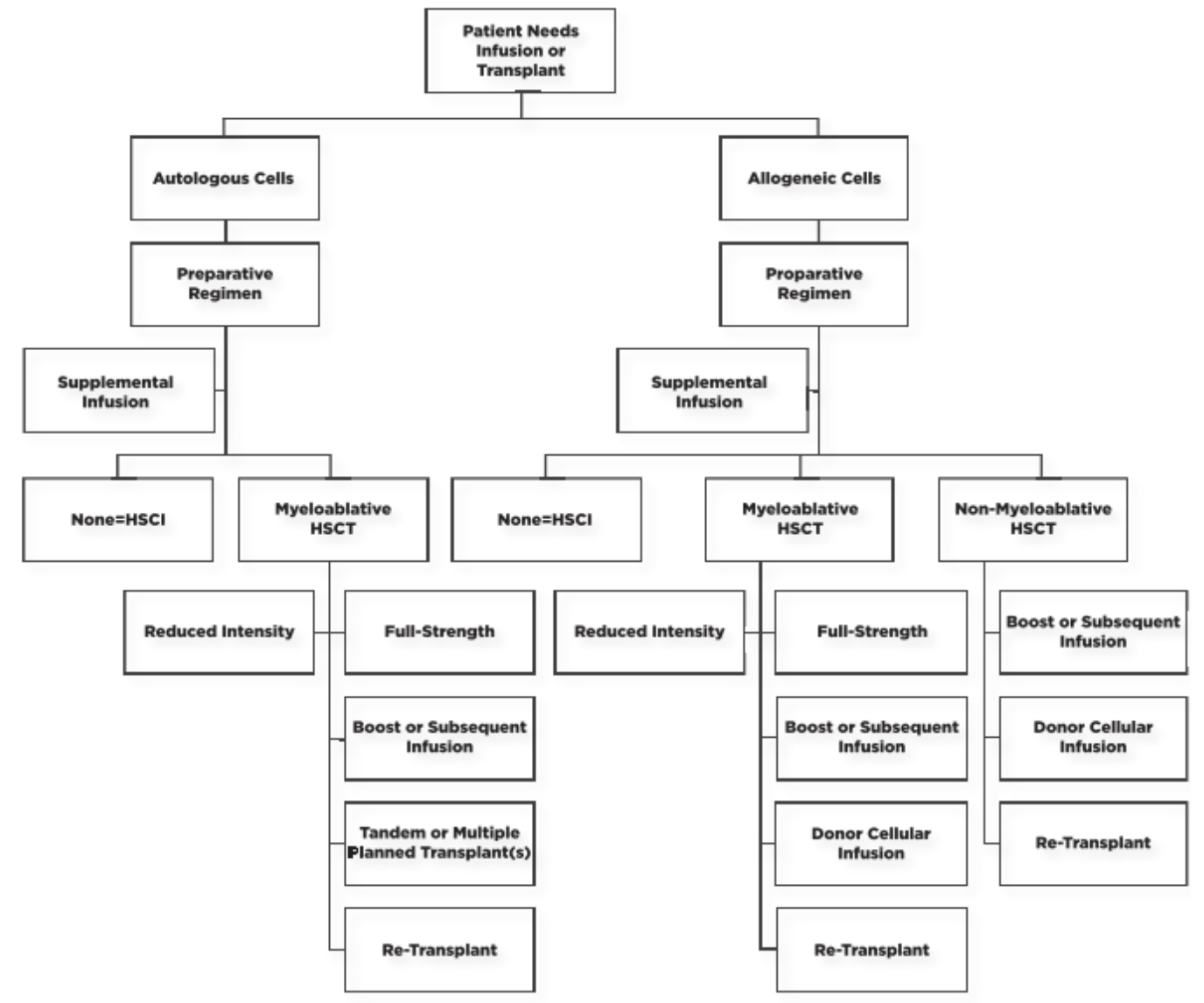
You can see from the diagram below how these terms somewhat fit together. Additionally, this figure does a great job of highlighting how complex HSCT can be when making clinical decisions for your patient.
(Image)
General Concepts of HSCT
All of the concepts that make up the basis of HSCT are based on the unique characteristics of the stem cell. When thinking back to our hematopoietic lineage, the stem cell is the top-most point, with it really being the starting point for all of our cells. Because of this, hematopoietic stem cells are able to produce daughter cells that retain their own properties – making them self renewing.
When comparing this cell proliferation concept to what’s going on in hematologic malignancies, it is actually quite similar because cancer is driven by rapid proliferation of malignant cells. So in those cancerous cases, there’s a daughter cell lineage that goes awry. However, in the case of HSCT, given the infused stem cells are non-cancerous at time of administration, they proliferate into non-cancerous daughter cells that are productive members of the body, performing their duties accurately.
Without going into too much detail, it is important to note some key concepts influencing HSCT success…
First, stem cells are incredibly resilient. Therefore, when chemotherapy is administered, a lot of stem cells are spared. This can allow for cancers to recur following transplant. So while we do like to think that this wouldn’t happen in this population, it unfortunately can occur.
Second, it’s important to note that, for patients who receive allogeneic transplants, the success of these transplants is related to the histocompatibility of the donor and recipient (similar to solid organ transplant (SOT)). Despite the patient receiving preparative regimens (which we’ll be discussing later), these patients still have lymphocytes that present antigens in their bodies at time of stem cell infusion. Therefore, after receiving the stem cell infusion, the recipient's native T-cells can interact with the surface major histocompatibility antigens (just like for any infection).
These native T-cells can recognize the donor’s antigens and thus reject the new graft. Obviously, this not favorable in this population, and we try to prevent this from happening by giving preparative chemotherapy.
Next, in addition to the patient’s lingering immune system even after chemotherapy, it’s important to note that the donor’s T-cells will also be present at this time [literally two immune systems in one host – whoa.]. The donor’s T-cells will act similarly as the recipient’s, but instead of targeting the new graft, these will act as if the recipient's native cells are foreign (think epithelial cells of different organs and these T-cells not recognizing them). This specific interaction is what causes graft-versus-host disease (GVHD) in our HSCT patients. This is one of our primary complications in the HSCT patient population and can have severe impacts on quality of life.
We’ve established why the patients’ major histocompatibility antigens are important factors to consider when matching donors to recipients. But it’s also incredibly important to consider the minor histocompatibility antigens as well. The specific role that these serve is to provoke a graft-versus-tumor response. Just like it sounds, this response is the impact that a stem cell graft can have on any remaining leukemic cells following the preparative chemotherapy regimen.
As stated earlier, stem cells are incredibly resilient and thus the malignant ones can sometimes hang around despite an adequate preparative therapy being administered. Therefore, this graft-versus-tumor effect is SO important for the success of that graft and to ensure that the patient has as little of a chance as possible for relapse.
When is HSCT Indicated?
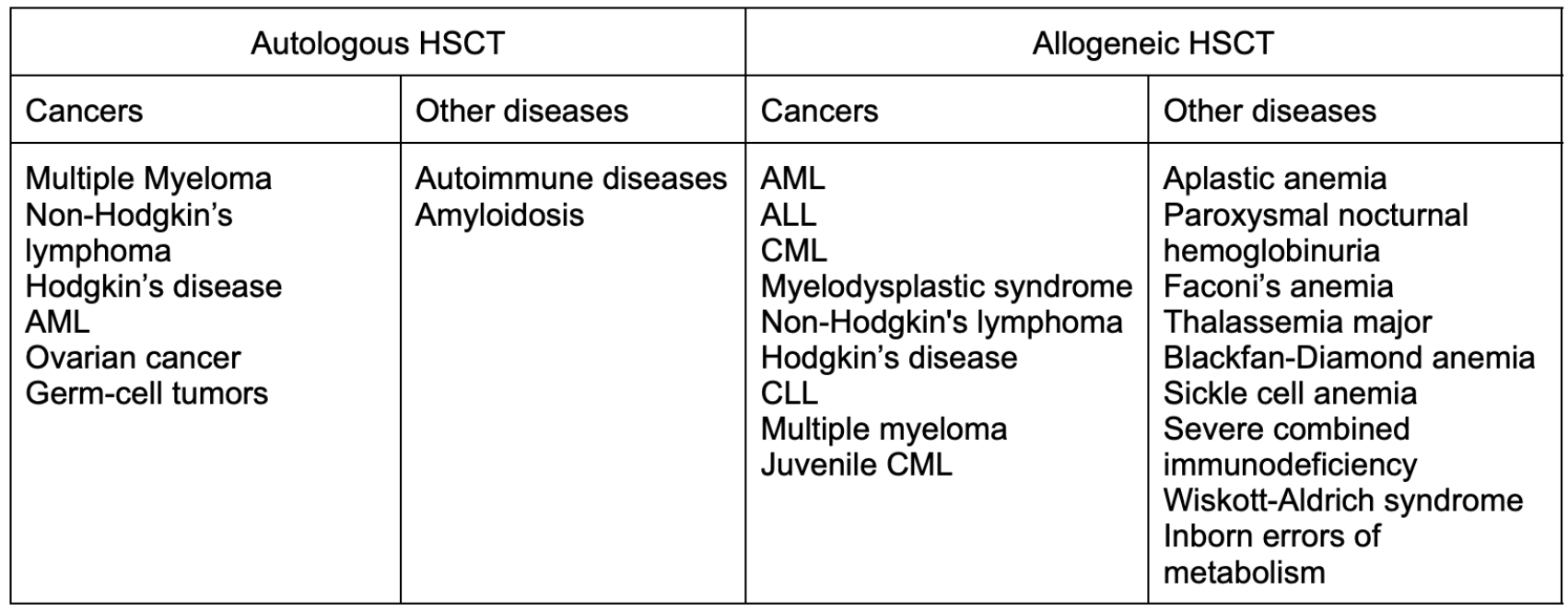
Now that we’ve reviewed the scientific concepts of this procedure, we can discuss the patients who benefit. As you can imagine, similar to SOT, HSCT replaces the patient’s bone marrow, which means it is primarily a treatment modality for patients who have some disease driven by a dysfunctional marrow. For some diseases, autologous HSCT is favored versus allogeneic HSCT. You can see these diseases below:
Fascinating fact… HSCT is also under investigation for multiple sclerosis!
For the diseases where either autologous or allogeneic is indicated, the decision is made based on patient-specific characteristics, assessing risks and benefits of each of these routes, and then having a shared decision making conversation with the patient. GVHD is not a concern in those patients who receive autologous HSCT due to the infused cells being the patient’s own marrow. However, the data for relapsing potential favors allogeneic HSCT if the patient is able to tolerate this procedure and the potential complications. Therefore, it is all a risk-benefit decision and really should be made with efforts to meet the patient’s overall goals of care.
Preparative Chemotherapy Regimens for HSCT
Chemotherapy agents used for HSCT conditioning.
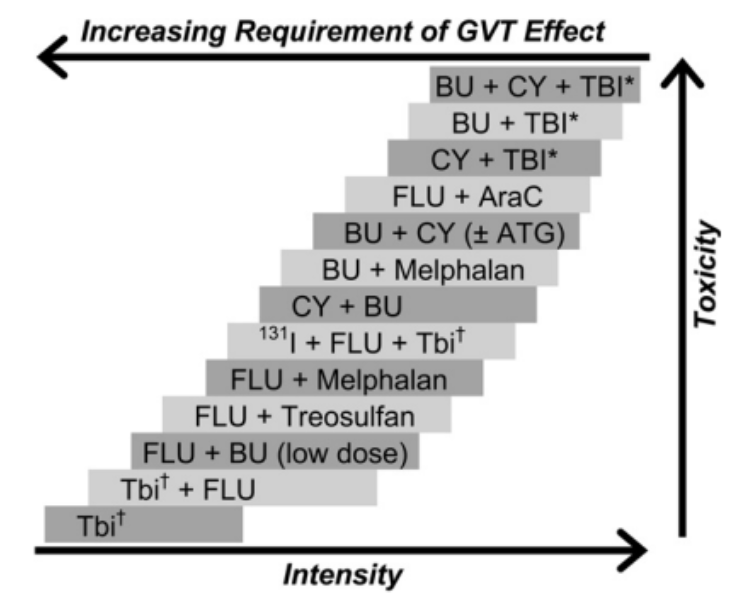
Preparative regimens are intended to ‘prepare’ the marrow with myeloablative (literally: cell-killing) chemotherapy prior to transplant. The overall goal of this bomb drop is to eradicate the cancer and induce immunosuppression that permits engraftment. The original preparative regimen used for patients was total-body irradiation (TBI); however, radiation-free regimens also exist due to toxicity and limited accessibility of this strategy. For this reason, many different preparative regimens are now used in the clinical setting.
(We also don’t always drop a total bomb. For some, there’s the option of non-myeloablative chemotherapy conditioning prior to transplant, which is known as a ‘mini-transplant’.)
Anyways, there really aren’t that many different types of chemotherapy used for conditioning regimens. However, that doesn’t mean this doesn’t get confusing! This is because the agents are used in so many different combinations…
Not only are they used in a wide array of combinations, these regimens can also vary in intensity. Each patient’s regimen should be individualized to their clinical picture. In general, reduced-intensity regimens are used in older patients or those with concerns for tolerability. Remember, the purpose of this conditioning is to eradicate the cancer and induce immunosuppression, so when administering these therapies, it is a VERY fine line that you walk to balance tolerability of chemotherapy with transplantation success.
Demonstration of the fine line between intensity, toxicity, and impact on GVT effect. (Image)
In addition to preparative regimens, an infusion of donor lymphocytes can be used to induce a graft versus tumor response. This is especially necessary for patients receiving lower intensity regimens, in hopes of allowing the graft to kill any remaining tumor cells present in the body (i.e., prevent disease relapse).
Sources of Stem Cells
There are four primary sources of stem cells that are used in HSCT:
cord-blood,
unrelated donor,
a related donor, and
the patient themselves (for autologous transplants).
Cord blood is generally used less often; however, this can be procured easily and safely if need be. The concept that drives the use of cord blood is based on the thought that the umbilical cord and placenta have a very high volume of stem cells. Therefore, these can be frozen after birth and then used as a source for transplant.
Notably, these cells are more slow-to-function following transplant, and thus infections are more common in our patients with cord transplants versus other types. However, cord blood is less stringent for HLA matching and can still have the GVT effect. That’s why it can be a good option for those without other options and with an acute HSCT need.
Relying on the match to find an unrelated donor may feel a bit like throwing up a Hail Mary… (Image)
Another transplant source to focus on is the haploidentical, or related, donor. The benefit of using a relative’s blood, whether from a parent, sibling, or child, is due to at least one HLA haplotype being similar to the recipient’s. When using this stem cell source, there is an additional alloreactive mechanism that occurs involving natural killer cells. These cells interact with the class 1 HLA epitopes, which are hopefully similar in this case. This alloreactivity improves engraftment and reduces GVHD risk. Therefore, this is obviously a preferred method, if possible. However, for the majority (~70%) of patients in need of an HSCT, a related donor isn’t on the table and so they have to rely on the match to find an unrelated donor to optimize pairing.
Complications of Stem Cell Transplant
As you might imagine, giving a patient high-dose chemotherapy and then replacing their bone marrow and entire immunologic system creates the potential for a plethora of complications. These issues are either directly caused by the procedure itself or can be due to the state of the patient’s immune system following the HSCT.
Early complications may include mucositis (due to the high-dose chemotherapy), as well as sinusoidal obstruction syndrome, transplantation-related lung injury, and transplantation-related infections. The most important complication of allogeneic transplant is GVHD.
There are two types of GVHD: acute and chronic. Acute GVHD generally targets the skin, gut, and liver. The standard of care for both acute and chronic treatment of GVHD is corticosteroids. The primary risk factor for GVHD is the presence of HLA mismatch, but notably, this can still occur with matched donors. Methotrexate and cyclophosphamide can be given with transplantation to hopefully prevent acute GVHD, but unfortunately this still cannot be guaranteed.
Check out those corticosteroid adverse effects… the list is HUGE! Pick an organ system, any organ system. (Image)
Similar to acute GVHD, chronic GVHD is a relatively common complication. As its name suggests, it is more delayed in these patients. Nonetheless, it is incredibly serious and can have an extreme impact on quality of life for HSCT patients. Chronic GVHD can impact many different organ groups, causing issues such as bronchiolitis, keratoconjunctivitis sicca, esophageal stricture, malabsorption, cholestasis, hematocytopenia, and generalized immunosuppression.
In these cases, steroids may be required for years at a time. Other medications can be used as second or third line therapies, but corticosteroids continue to remain the gold standard.
Other notable long-term compilations of HSCT include secondary cancers. Similarly to SOT, due to the body's chronically immunosuppressed state, it is no longer able to fight off cancers on its own. Therefore, patients are at a higher risk, especially for skin, oral mucosa, brain, thyroid, and bone cancers.
CAR-T versus HSCT
The last thing we’re going to cover today is CAR-T therapy. HSCT and CAR-T are frequently confused due to both of these being indicated for very similar disease states. Plus, they’re usually administered on the same unit. Therefore, it’s important to discuss what CAR-T is and how this differs from HSCT.
Is it just me, or is it pretty ridiculous (and unbelievably cool) that this is even possible? (Image)
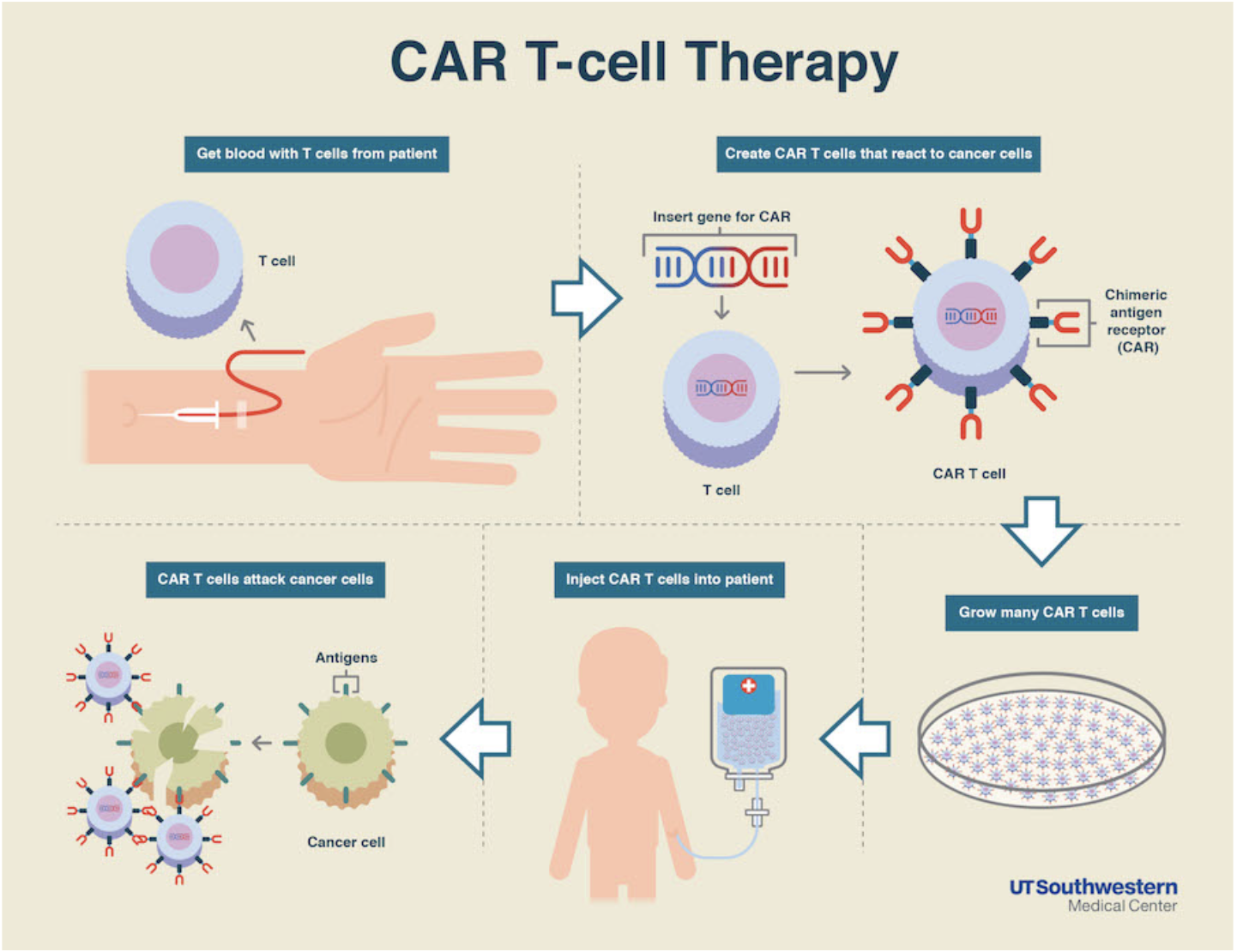
CAR-T (aka Chimeric Antigen Receptor T-cell) therapy is a revolutionary strategy that involves taking a patient’s T-cells, priming (or training) those T-cells to recognize the patient’s cancer, and then re-infusing these trained mercenaries, allowing them to target the patient’s malignancy.
While similar to an autologous transplant in that this modality uses the patient’s own blood, it’s different in that the patient doesn’t technically require a preparative regimen. That’s because the primed T-cells act as the chemotherapy in this case.
Additionally, CAR-T is different from HSCT because it isn’t as profoundly immunosuppressive as HSCT is. It also can be used for diseases outside the bone marrow! Currently, CAR-T is primarily used for certain types of B cell leukemias or lymphomas; however, multiple myeloma just recently had CAR-T approved by the FDA.
Plus, CAR-T is potentially a therapeutic option even beyond cancers (think - maybe cardiac fibrosis or multiple sclerosis!?).
Despite this avenue having a lot of appealing factors, it is not without its limitations. The biggest limitations are really the cost barrier and the lack of current published data. CAR-T’s place in therapy is still being debated, and with this being such a labor intensive and patient-specific therapy, the entire process of CAR-T therapy can cost hundreds of thousands of dollars (hopefully not for the patient but insurance determines that).
Additionally, CAR-T therapy is not without its adverse events. Patients can have very different presentations than the ‘standard’ chemotherapy complications since this therapy is far from standard! Associated toxicities include cytokine-release syndrome and neurotoxicity. Due to CAR-T therapy being so new and innovative, we’re still waiting on publication of robust data regarding the management of these adverse events. However, with time, CAR-T will continue to grow, will continue to make its presence even more known, and will hopefully be an answer (in addition to HSCT) for patients in need of accessible cancer care.
The tl;dr of Hematopoietic Stem Cell Transplant
HSCTs are a useful - but grueling - treatment modality for treating multiple types of hematologic malignancies. Plus, they can be used for certain non-cancer indications as well. We will need to continue to follow the CAR-T saga to see how this new and innovative therapy relates to HSCTs with regards to efficacy, adverse effects, and cost-effectiveness.
And remember, if you haven’t already, register for Be the Match!! NOW!!! Spread the word!!