An Introduction to Acute Coronary Syndrome
Steph’s Note: We’ve covered several cardiology topics over the years in previous posts, including heart failure and LVADs, but we (and our guest posters) never quite mustered the courage to tackle Acute Coronary Syndrome. Until today. Today we bring you the tl;dr overview of ACS!
This is all possible with the help of an eager volunteer author with a passion for cardio. Kelsey Dorka is a P4 student at the University of Toledo College of Pharmacy and Pharmaceutical Sciences. Kelsey’s career goals include a PGY1 residency, teaching certification, and PGY2 followed by a clinical position in NW Ohio. Her professional interests surround cardiology, infectious diseases, advocacy/pharmacy law, and ambulatory care. Outside of pharmacy, Kelsey enjoys spoiling her 3-year-old boxer dog named Penny and watching The Office re-runs.
Acute Coronary Syndrome (ACS)
Cardiovascular disease is an umbrella term that encompasses these 3 components. (Image)
The heart - everyone has one (despite popular belief), and everybody needs a functioning one. Unfortunately, the leading cause of morbidity and mortality in the United States is cardiovascular disease (CVD). Furthermore, coronary heart disease (CHD) is the most common reason for cardiovascular death.
Breaking this down further, one manifestation of CHD is acute coronary syndrome (ACS), which includes: myocardial (fancy word for heart) ischemia (fancy term for the restriction of blood supply and oxygen to tissues) – as well as myocardial infarction (MI) (aka a “heart attack”).
Pathophysiology of Acute Coronary Syndrome (ACS)
The coronary arteries Snuggie (Image)
ACS most commonly results from the obstruction of blood flow in a coronary artery. This obstruction prevents oxygen from reaching the most important organ in your body - the one that keeps you upright and continually reading tl;dr blog posts late at night before exams. For real, your heart requires that continual flow of oxygen to function and survive, similar to any other organ or tissues. Your coronary arteries wrap around the entire heart, like a warm oxygen-rich hug or a “snuggie,” to make sure every part of the heart muscle and tissue gets what it lovingly deserves.
So how does obstruction happen?
Obstruction can be the result of a rupture or erosion of vulnerable, lipid-rich, atherosclerotic plaque, leading to a thrombus (or clot) that partially or completely blocks a coronary artery.
Atherosclerotic plaque is the result of endothelial cell dysfunction in the intima (innermost layer of arterial wall) and is enhanced by hypercholesterolemia. These plaque lesions are made up of smooth muscle cells, lipid material, macrophages, foam cells, T lymphocytes, connective tissue – and probably anything else you could find in your kitchen’s junk drawer. As this plaque grows and protrudes into the lumen, it can compromise blood flow, weaken the arterial wall (hello impending aneurysm), or eventually become susceptible to injury, leading to plaque rupture and possibly allowing a clot to form. Again, the return of the evil clot!
So who’s at risk for ACS?
Speaking of risk factors…
If a patient fits into any of these descriptors or confirms any of these lifestyle habits, it could be beneficial to determine just how much risk they have of ACS. And because of technological advances and existing at just the right moment in time, we can use a great tool in our clinical backpack:
The handy-dandy, completely free courtesy of ACC/AHA, ‘Atherosclerotic Cardiovascular Disease (“ASCVD”) Risk Calculator’ (even available as a smart phone app!).
If you haven’t played the Exploding Kittens card game, you should. It’s delightfully random. (Image)
This calculator is for patients with an LDL less than 190mg/dL (not currently on LDL lowering therapy) and without established ASCVD (or else they would already know they are at risk and this calculator would be a moot point).
But why do we care about something that may or may not happen later? (Psssst… PROPHYLAXIS). Let’s say you were allowed to play your “see the future” card (you know – from that game Exploding Kittens). What would you do with that knowledge? Would you use it to determine which pharmacotherapy your precious cat-loving grandma should be starting today in order to prevent a cardiovascular event, say, maybe tomorrow?
This calculator’s superpower may save a life.
After you plug in the necessary information and answer all the prompts, the estimator calculates a 10-year and a lifetime ASCVD risk score (%) but then also uses its own algorithm to spit out a recommendation – which you must carefully consider using your clinical judgement, of course.
For funsies (and to help y’all remember those risk factors in an alternative way), let’s break down the ASCVD Risk Calculator according to those risk factors we just discussed above:
Play around with the calculator to see for yourself how different risk factors influence the risk outcome of a patient.
Signs and Symptoms of ACS
Remember that we defined ischemia as a lack of blood flow. If muscle, including cardiac muscle, goes long enough without sufficient blood flow, this can lead to myocardial necrosis, or cardiac muscle cell death.
Bad, right? Yes. Painful? Yes, specifically chest pain.
This is often described as a pressure/squeezing sensation lasting more than 10 minutes (the “elephant on your chest” feeling) and can be accompanied by difficulty breathing, profuse sweating, syncope, and heart palpitations. Pain may radiate to the arms, back, neck, jaw, or esophagus area, sometimes making it tricky to distinguish from other medical issues like GERD. ACS chest pain can be experienced at rest or as a result from minimal exertion, exercise, cold weather, extreme emotions or stress, and even sexual intercourse.
Diagnosis of ACS
The various subcategories of ACS are differentiated by the presence of cardiac enzymes, changes observed on an ECG (electrocardiogram, aka EKG), and extent of blockage to the artery in question.
Cardiac Enzymes in ACS
Cardiac enzymes are released into the bloodstream when myocardial cells die. Cardiac troponin I and cardiac troponin T are the most sensitive and specific biomarkers for ACS and should be measured ASAP when the patient first presents and again at 3 to 6 hours (and sometimes again even up to 12 – 24 hours) after symptom onset to determine the rising or falling pattern.
The presence and magnitude of these levels can be useful for short and long term prognosis and may also be measured yet again 3 or 4 days later in patients with MI to determine the infarct size and dynamics of necrosis.
ECG Changes in ACS
A 10 or 12-lead ECG should be taken within 10 minutes upon patient’s arrival to a hospital to assess for ischemia or injury.
Sometimes it seems like wizardry when trained medical personnel look at scribbled ECG printouts and then declare a diagnosis. When you look at the printout later, it’s like a Where’s Waldo of what clues tipped them off. Reading ECGs is an art form, but just like everything else, it is a learned skill. Basically, each lead corresponds to a different spacial orientation, and when taken all together, trained clinicians can get a pretty darn good picture of where and what types of damage have occurred.
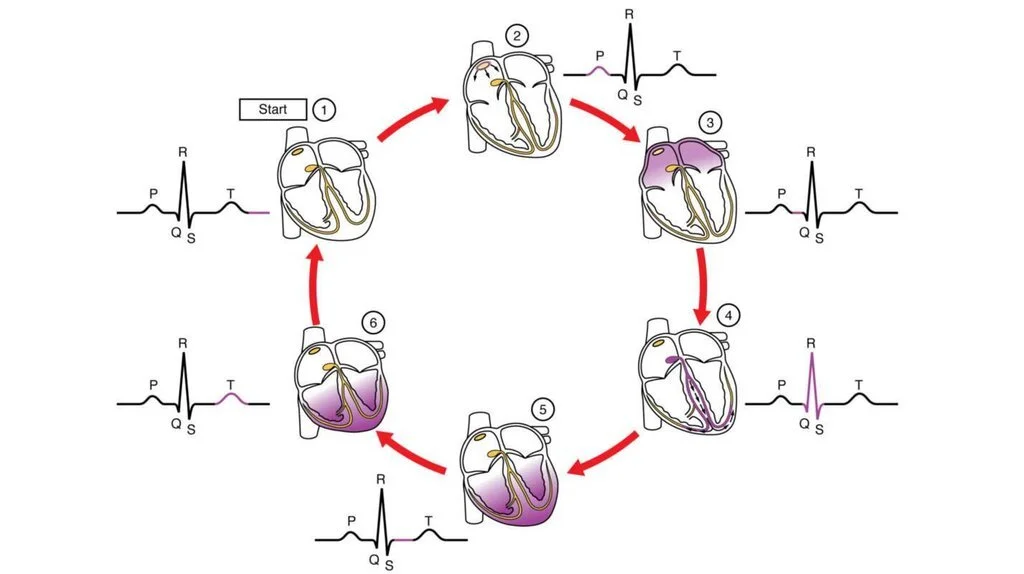
Let’s take a closer look at the normal cardiac cycle and ECG. Starting with #2, atrial depolarization occurs starting with the SA node, which causes the P wave. #3 (the interval between the P wave and the QRS complex) depicts the pause that occurs as the electrical signal is regulated by the AV node, between the atria and ventricles. The signal then passes through the AV node down into the ventricles, with ventricular depolarization occurring starting in the apex of the heart. Ventricular depolarization is represented by the QRS complex (#4). This is also when atrial repolarization happens, but it’s kind of hidden in the QRS complex. #5 represents the completion of ventricular depolarization (the interval between the QRS complex and the T wave). Then the ventricles repolarize, as visualized with the T wave. #1 is the period between ventricular repolarization and atrial depolarization. (Image)
In case you’re interested in attending Hoghearts School of ECG Wizadry (or you just have an upcoming exam on ECG tracings), here’s a super useful site to give you a leg up on your training.
Now that we’ve walked through the normal parts of the ECG, what do we mean by abnormal ECGs in ACS?
Examples of ECG findings in 2 STEMI cases. Notice how each lead (aVL, II, aVF, etc) tracing looks different and how the clues from the various leads have to be melded to make a diagnosis. (Image)
Persistent ST-elevation or anterior ST depression indicate a true posterior MI (STEMI). Changes on ECG for NSTE-ACS (formerly known as NSTEMI) include ST depression, transient ST elevation, or new T- wave inversion. If the initial ECG is normal but the patient remains symptomatic, multiple ECGs should be performed every 15 to 30 minutes during the first hour to monitor for changes.
One big takeaway - if you observe elevation in the ST-segment, it could be safe to assume you are dealing with a STEMI.
Acute Management of ACS
Acute management goals are to provide immediate relief of ischemia, limit infarct size, and prevent death.
So how do we accomplish these pieces?
Step 1: Cool Down the Heart with “MONA-B”
The goal of this acute supportive care strategy is to decrease myocardial oxygen demand and increase oxygen supply (blood flow) to relieve ischemia.
More blood = more oxygen = happier heart muscle.
Morphine: This opioid causes arterial and venous dilation (↓ in both preload and afterload means ↓ cardiac workload), and it also reduces chest pain.
Anti-anginal administration: Morphine sulfate 2 – 5mg IV repeated at 5 to 30-minute intervals as needed for chest pain.
Oxygen: Flow at 2 – 4 L/min should be given when arterial O2 saturation (saO2) is less than 90% or when a patient is experiencing respiratory distress.
Nitrates: These medications dilate coronary arteries and improve collateral blood flow by ↓ preload and afterload (modestly), and they also reduce chest pain. Contraindications to use of nitrates include recent use of oral phosphodiesterase-5 (PDE-5) inhibitors like sildenafil.
Sublingual nitroglycerin 0.3 – 0.4mg every 5 minutes for up to 3 doses as needed for chest pain.
If relief is insufficient, may transition to a nitroglycerin IV infusion at 5-10 mcg/minute and titrate ↑ by 5mcg every 5-10 minutes for persistent chest pain. Initial increases are made up to 20mcg/minute, but if angina persists at a dose of 20 mcg/minute, the rate may be increased by 10 to 20 mcg/minute every 3 to 5 minutes to a maximum dose of 400 mcg/minute.
Aspirin: This irreversible COX1 and COX2 inhibitor decreases platelet aggregation and subsequent clot formation by inhibiting production of thromboxane A2 (TXA2).
Chewable aspirin 162 – 325mg should be administered to all patients immediately (if not contraindicated). Extended-release aspirin products should NOT be used.
Continue aspirin 81 – 162mg daily indefinitely (hold tight for post-discharge/secondary prevention in a few paragraphs).
Beta-blocker: Metoprolol or carvedilol should be initiated within the first 24 hours for their benefits of ↓ cardiac workload.
Carvedilol 6.25mg orally twice daily. Titrate to a target dose of 25mg twice daily as tolerated.
Metoprolol 25 – 50mg by mouth every 6 to 12 hours for 2 to 3 days, then once daily (as the succinate formulation) or twice daily (as tartrate). Titrate to a target dose of 200mg daily.
If necessary, metoprolol may be administered in IV form. The dosing for IV is a little less than its PO counterpart (IV to PO ratio is somewhere in the 1:2.5 to 1:5 range). For specific cases such as some STEMI patients with acute uncontrolled hypertension or refractory symptoms, metoprolol 5mg IV may be given as frequently as every 5 minutes as tolerated up to 3 doses, titrated to BP and HR control.
Step 2. Classify the ACS Syndrome
We previously mentioned STEMI in the ECG section of this post, and we discussed what features on an ECG qualify a patient for this diagnosis. But what’s actually going on behind the scenes to cause these more extreme, very characteristic ECG changes?
STEMI is characterized by a plaque rupture that causes a complete and persistent blockage of one or more coronary arteries. This leads to ischemia and infarction of the entire myocardium. Characteristics include chest pain, plaque rupture, ST-segment elevation on ECG, and positive labs for cardiac markers (Troponins I and T).
In contrast, NSTE ACS+ (previously known as NSTEMI) is characterized by a plaque rupture that causes a partial blockage of cardiac blood flow. This leads to ischemia and infarction of the inner layer of the ventricular cell wall. Characteristics include chest pain, plaque rupture, no change/depression of ST-segment on ECG (with notable T-wave inversion), and positive labs for cardiac markers. Angiography (aka a cardiac catheterization) should be used to determine the sites of blockage and whether further management should take a more aggressive versus a more conservative approach (e.g., PCI vs. medical management).
Which leads us to our next section… Surgical +/- pharmacologic interventions.
Step 3: Determine a Reperfusion Strategy
The first decision point for re-establishing and maintaining cardiac blood flow is PCI vs thrombolytics. Although they are accomplished using different tools, both of these are essentially manual fixes to unblock the affected blood vessels - like calling the plumber when your shower drain gets clogged.
PCI in action. Seems so simple…but so genius. (Image)
Percutaneous Coronary Intervention (PCI) is a revascularization procedure that involves inflating a small balloon inside a coronary artery to widen it and improve blood flow. A metal stent is put into place to keep the artery open. Primary PCI of the ischemic area within 90 minutes of hospital arrival is preferred over fibrinolytic therapy when PCI-capability is available to appropriate STEMI patients.
If, however, PCI is not possible within the first 120 minutes, then fibrinolytic therapy should be started within 30 minutes of arrival. The team can also consider transferring the patient to a PCI-capable institution within 24 hours to improve outcomes.
Fibrinolysis involves the use of thrombolytics to lyse (dissolve) already formed clots when the anticipated time to PCI is expected to exceed 120 minutes. Fibrinolytic therapy should be given within 30 minutes of hospital arrival provided no contraindications are present. Plasminogen activators start the process of fibrinolysis – the breakdown of fibrins which hold a clot together. In this case, breaking down the fibrin crosslinks is a good thing because it helps to restore blood flow by “dissolving” the clot.
There are a number of fibrinolytics available in the US today, including the following:
Alteplase 15mg IV push over 1-2 minutes, followed by 0.75mg/kg (max 50mg) IV over 30 minutes - and then 0.5mg/kg (max 35mg) IV over 60 minutes (maximum total dose 100mg).
Reteplase 10 units IV push over 2 minutes, followed 30 minutes later with another 10 units IV bolus over 2 minutes.
Tenecteplase is given as a single IV bolus over just 5 seconds. The mg dose is based on patient weight.
Streptokinase is not fibrin-specific and is therefore not preferred when compared with the aforementioned choices.
You can probably imagine that the biggest risk of using fibrinolytics is bleeding. While PCI comes with its own set of risks, including contrast-induced nephropathy and stroke, it is a relatively localized fix. PCI is an example of the plumber narrowing down exactly where the clog is and fixing only that part of the pipe. Fibrinolysis, on the other hand, is like pouring multiple cans of Drano into the shower drain, sitting back, and waiting for the chemicals to run through the system.
On this train of thought, one major takeaway is that if the chosen reperfusion strategy involves a fibrinolytic, this will influence the choice of the second antiplatelet agent further down the management chain (hold, please).
(Image)
Coronary Artery Bypass Graft (CABG) surgery is yet another reperfusion option. This procedure (often historically known as “open heart surgery” or “bypass surgery”) detours the blocked portion of the coronary artery by using a piece of healthy blood vessel. This healthy blood vessel is actually harvested from elsewhere in the patient’s body, such as the LEG… way cool, right?.
It is attached to points above and below the blockage, and blood then travels through the “new” roadway to reach the previously deprived heart muscle. CABG is usually the option when PCI and medications cannot sufficiently treat a blockage.
Step 4: Maintain the Newly Re-established Perfusion
Now that the heart muscle is happily receiving oxygenated blood again and is beginning to try to recover from the ischemic insult, it’s time to consider how that clear roadway should be maintained. This is done with antithrombotic therapy, which is a combination of antiplatelet + anticoagulant medications.
So first, let’s tackle the antiplatelets. In terms of ACS management, antiplatelet therapy actually refers to Dual Anti-Platelet Therapy (DAPT), as in we actually give more than one medication to inhibit platelet aggregation (whoa, intentional duplicative action?!?). DAPT usually consists of aspirin + a P2Y12 inhibitor, which help to prevent clot formation or growth during reperfusion therapy and also reduce the risk of stent thrombosis.
As mentioned previously in the MONA-B section, barring some occasional exceptions, aspirin is basically used in all ACS patients. The loading dose of 324mg (4 x 81mg chewable tablets) comes first, followed by 81mg daily indefinitely thereafter. Ideally, the maintenance dose is achieved using the enteric-coated formulation to help protect the stomach lining and prevent GI bleeding issues, but if a patient is unable to take medications by mouth, the regular release formulation can be useful to administer per tube.
As far as the selection and dose of the second antiplatelet agent to comprise DAPT, the previously determined reperfusion strategy (e.g. PCI vs fibrinolytic) influences this decision. (Be sure to pay special attention to the antiplatelet’s loading dose recommendations when researching recommendations for your patients!) The second antiplatelet agent is either a member of the P2Y12 inhibitor class or the glycoprotein (GP) IIb/IIIa inhibitor class.
P2Y12 inhibitors are sometimes also known as adenosine diphosphate (ADP) receptor antagonists. These drugs (or their active metabolites) bind the ADP P2Y12 receptor located on platelets. This is a G-coupled protein receptor (GPCR) with downstream effects that normally enhance the process of platelet aggregation by changing the ability of the GP IIb/IIIa receptor complex to bind its ligand. But when we block the P2Y12 receptor’s actions with these medications, the end result is reduction in platelet aggregation.
There are 2 different types of P2Y12 inhibitors, thienopyridines and nonthienopyridines. Thienopyridines irreversibly inactivate the platelet P2Y12 receptor, making it inactive for the life of the platelet. In contrast, nonthienopyridines reversibly inhibit the platelet P2Y12 receptor.
Clopidogrel and prasugrel are the thienopyridines, whereas ticagrelor and cangrelor are nonthienopyridines given their reversible inhibition of the the P2Y12 receptor. (Image)
Now on to the second possible DAPT class of medications, the GP IIb/IIIa inhibitors.
Two roads diverged in a wood… and the GP IIb/IIIa receptor antagonists block binding of ligands like fibrinogen to activated platelets. (Image)
These medications target the final common pathway of platelet aggregation by blocking the GP IIb/IIIa receptor binding site for fibrinogen, von Willebrand factor, etc. They are usually most useful in either STEMI patients managed with PCI or as adjuncts to PCI in NSTE-ACS+. A GP IIb/IIIa inhibitor is not routinely used due to limited benefit on ischemic outcomes and the increased bleeding complications. However, use may be considered in high-risk patients when PCI is planned.
A few tidbits about the members of this GP IIb/IIIa inhibitor class:
Discontinued in the US as of September 2019, abciximab was used as an adjunct to unfractionated heparin at time of PCI. It was administered as a 0.25 mg/kg IV bolus given 10 – 60 mins prior to start of PCI followed by an infusion of 0.125 mcg/kg/minute (maximum: 10 mcg/minute) for 12 hours. (You can see with those units changes why order sets can be so crucial with these time-sensitive medications!)
Eptifibatide is preferred over abciximab in NSTE-ACS+ patients treated with PCI. It is also given as a bolus followed by an infusion, but importantly, the dose must be adjusted for renal dysfunction.
Tirofiban is the third option in this class. Much like eptifibatide, it is given as a loading dose (25 mcg/kg administered over 5 minutes or less) followed by a maintenance infusion of 0.15 mcg/kg/minute continued for up to 18 hours. It must also be adjusted for renal dysfunction.
These GP IIb/IIIa inhibitors should be used with great caution - if at all - after full-dose fibrinolytic therapy because this combination is associated with high rates of bleeding and intracranial hemorrhage, particularly in the elderly.
Ok, so to briefly review. At this point, we’ve discussed medications for fibrinolysis as well as the DAPT portion of antithrombotic therapy. So what’s left? The anticoagulant portion of antithrombotic therapy, which is where we’ll go next!
Step 5. Smash Coagulation with Adjunctive Anticoagulants
Anticoagulants are medications that inhibit clotting factors to prevent clot formation or growth in order to maintain hemodynamic control. Current guidelines recommend antithrombotic therapy (aka the combination of DAPT with anticoagulant therapy) for hospitalized patients with ACS for up to 48 hours after the event or until PCI is performed.
Although there are many MANY medications on the market that are classified as anticoagulants, not all of them are options for use in the acute phase of ACS. Let’s take a moment to investigate the ones that are.
Note how UFH is a much larger molecule that is able to impact both thrombin and factor Xa, whereas the chopped off tail of LMWH is really only able to affect factor Xa activity (with maybe a smidge of thrombin). Then the pentasaccharide is only able to impact factor Xa, no thrombin at all. (Image)
Unfractionated heparin (UFH) is recommended in STEMI patients undergoing primary PCI or fibrinolytic therapy, and the dose is adjusted to maintain the activated partial thromboplastin time (aPTT) at 1.5 to 2 times normal for 48 hours and until revascularization (up to 8 days or for the duration of hospitalization).
Enoxaparin is a low-molecular weight heparin (LMWH) that is recommended in STEMI patients undergoing fibrinolytic therapy. It is preferred over UFH in NSTE-ACS+ patients managed with either reperfusion strategy. Remember that enoxaparin has to be adjusted for renal dysfunction!
ACS is one of the only times you will ever see orders for IV enoxaparin! Otherwise, this order should make your spidey sense activate, for sure. But for ACS patients less than 75 years old, a 30mg IV bolus is administered first. Then 15 minutes later, enoxparin 1mg/kg subcutaneous injections are initiated and continued every 12 hours (btw, the first 2 doses are capped at 100mg - I mean, we did just give an IV bolus of the stuff and they are potentially undergoing a procedure and receiving other bleed risk medications…).
For patients aged greater than 75 years, SKIP that bolus. No no. Instead, just give 0.75 mg/kg subcutaneously every 12 hours (max 75mg for the first 2 doses).
Fondaparinux is a synthetic pentasaccharide that may be used for treating NSTE-ACS+ patients with a high bleeding risk. It is not recommended as the sole anticoagulant for STEMI patients undergoing PCI because of catheter-related thrombosis seen in clinical trials. It is contraindicated when CrCl is less than 30 ml/min, so there’s not even the option of a dosage adjustment like there is with enoxaparin.
It is dosed as 2.5mg IV on day 1, then 2.5mg subcutaneously daily thereafter for up to 8 days or until revascularization.
Bivalirudin is a direct thrombin inhibitor (DTI) recommended in STEMI patients with high bleeding risk undergoing PCI. It is preferred over the use of UFH in combination with a GP IIb/IIIa inhibitor. It too requires adjustments for renal dysfunction.
It is given as a 0.75mg/kg IV bolus, followed by a 1.75mg/kg/hour infusion.
So that’s pretty much the main medications that you may encounter for acute management of ACS patients. It’s a LOT, we know! Hallelujah for order sets, although it’s always a good idea to know how the pieces of this puzzle go together (even if you don’t know all the bolus and infusion rates off the top of your head). You should at least be familiar with what kinds of medications are administered at what point in the ACS time course, as well as how those medications work to either open up a blood vessel or keep it open after blood flow is re-established.
Next, what happens to the patient’s medication list when he is ready for discharge? How do we prevent ACS from happening again?
Secondary Prevention of ACS
Although this could easily be an entire post itself, we’re going to briefly introduce this topic, just to round out the medication story. Hopefully, some brave soul will volunteer and email us because they love cardiology and want to review the literature behind these therapies so we know why we do what we do (HINT HINT, you know how to reach us!).
Anyways.
The easiest way to remember the medications used for secondary prevention is to think of a now defunct, formerly pricey car company: the Saab. ACS patients should go home “SAAB”ed-up, i.e., with a Statin, Aspirin, ACE inhibitor, and Beta-blocker.
High-intensity statin therapy has proven beneficial. This means atorvastatin 40 - 80mg daily or rosuvastatin 20-40 mg daily. The exception to this is in the elderly, for whom moderate-intensity doses may be acceptable.
Aspirin, aspirin, aspirin, FOREVER. Aspirin 81mg daily has been well-documented to extend life (aka it has a “mortality benefit”). So barring extreme hypersensitivity (and even desensitization to aspirin is sometimes a possibility!), just plan on all ACS patients being on aspirin.
ACE-Inhibitors or angiotensin-receptor blockers (ARB) (or perhaps even sacubitril/valsartan) are used to slow the atherosclerotic process and improve survival after ACS.
Beta-blockers like metoprolol and carvedilol (aka those without intrinsic sympathomimetic activity (ISA) activity!) extend survival after ACS.
Patients should probably also go home with a prescription for sublingual nitroglycerin (NTG), which can be used as needed in the event of chest pain (angina) symptoms. If and when patients experience chest pain, they can place one sublingual tablet under the tongue and allow the tablet to dissolve without swallowing. One additional tablet may be taken every five minutes for up to three doses for relief of chest pain. If chest pain/discomfort is not improved or is worsening five minutes after the first dose, it’s officially time to call 911.
Sublingual nitroglycerin can be supplied in three strengths: 0.3mg, 04.mg or 0.6mg tablets. Counseling point: patients should not crack the seal on their teeny brown bottles of nitroglycerin until a dose is needed. Once the seal is cracked, the remaining tablets are susceptible to moisture. Patients should know to replace their nitroglycerin supply about 3-6 months after opening.
Although some studies range all the way up to 12 - 24 months’ stability depending on how patients store their open bottles, when you need some good nitroglycerin, you need some good nitroglycerin… So it’s probably just better to replace it than risk having impotent nitroglycerin during an acute anginal attack!
Other Considerations for Post-ACS Pharmacotherapy
Patients may experience musculoskeletal pain following ACS. Options for pain relief include acetaminophen, non-acetylated salicylates, tramadol, or small doses of narcotics. Please run through all of these options before even thinking about suggesting NSAIDs! If absolutely necessary, it is reasonable to use nonselective NSAIDs (naproxen has the lowest cardiovascular risk). Definitely don’t forget that COX-2 selective NSAIDs have the highest cardiovascular risk and should be avoided.
For patients who have concomitant ACS as well as atrial fibrillation, there’s often the question of how to achieve the DAPT required for stunting after PCI plus the anticoagulation needed to prevent strokes from the atrial fibrillation. Dual or triple antithrombotic therapy may be an option. Yes, triple therapy with aspirin, an antiplatelet, AND an anticoagulant. As you can imagine, when using triple therapy, it should be for the shortest time possible, and clopidogrel is the preferred P2Y12 inhibitor in this scenario. Transitioning to dual therapy (anticoagulant + P2Y12 inhibitor) can be done after 4 to 6 weeks.
Finally, all ACS patients should be assessed for potentially applicable lifestyle counseling. Don’t forget to consider smoking cessation, proper management of chronic comorbidities like diabetes and hypertension, avoiding excessive alcohol intake, and encouraging physical exercise paired with a healthy diet.