A Thorough Note on Nuclear Pharmacy
Definitely how I felt opening Isabella's email! (Image)
Steph’s Note: This week, our post comes from a happy email that literally just showed up in my inbox. Like a bag of Reese’s dropped into my electronic lap, this post brings me so much joy! It’s new. It’s fresh. It’s something we haven’t ever DARED to touch. And our author wrote it (beautifully, I might add) purely because she noticed the void on our site. Call that motivation, right?!
Who is our dedicated author this week? Isabella Tobin is a pharmacy student at Purdue University interested in pursuing a career in nuclear pharmacy after graduation. She is from Rhode Island and misses the ocean when attending school in a landlocked state. Isabella also thanks her mentor, Luke Brissette, for his input on her magnum opus here. Dr. Brissette received his PharmD from the University of Connecticut. He's been preparing radiopharmaceuticals for CT, MA, and RI since 2020. Currently, he is a staff nuclear pharmacist, preceptor, and clinical trial liaison for a nuclear pharmacy in Rhode Island. If you’re interested in learning more, Dr. Brissette has graciously included his contact info: Luke.brissette@cardinalhealth.com.
Let’s dive in!
Anyone else learn everything they know about radiation principles from that one chapter in their high school chemistry class? It was maybe one week of lessons (at most), but I was fascinated. We learned that energy increases as wavelength decreases and frequency increases. We examined the spectrum: gamma waves, X-rays, ultraviolet, visible light, infrared, microwaves, radio waves (also known as Good X-Men Undo Violence in Magneto’s Regime).
But that was it.
I got a brief taste of this amazing science, and then they were going to take it away? I used my prodigious googling skills to teach myself more, and in my quest for knowledge, I discovered nuclear pharmacy. Since then, I have been apprenticing in a nuclear pharmacy and learning the duties of the pharmacists who work here.
If you think you might be interested in following the same path, or if you just want to learn more about radioactive pharmaceuticals, this post is for you. So grab your dosimeters because here comes your crash course in nuclear pharmacy.
What’s unique about radiopharmaceuticals?
Now, journey back with me to the days of chemistry, and think about the periodic table. It is full of elements. (Yeah, I know. Duh.)
All elements can exist in nature in multiple forms, or isotopes. The number of protons determines what an element is, but the number of neutrons determines stability.
Radioactive isotopes are unstable. They give off energy in order to reach stability. That’s what makes them radioactive. The energy they emit is often measured in millicuries (mCi) or curies (Ci) - one thousand mCis.
Types of Radiation
In order to prepare radioactive drugs, you first have to know about radiation. There are three main types of radiation we use in medicine: alpha, beta, and gamma. (No, you cannot become The Incredible Hulk from the gamma rays of working in a nuclear pharmacy. I was disappointed, too.)
Alpha particles are the biggest form of radiation. They’re made of two protons and two neutrons. I like to think of them as bumble bees…they’re pretty slow, don’t travel very far, and are the most easily blocked particle. If you hold an alpha particle in your hand, it won’t travel below your skin. A piece of paper can block an alpha particle.
Beta particles are smaller than alpha particles. They’re either positive (releasing positrons) or negative (releasing electrons). If alpha particles are bumble bees, beta particles are wasps. They’re more hazardous, penetrate through skin, and you need equipment to contain them. Aluminum, wood, and plastic can all block beta particles.
Gamma rays are the third type of radiation. Unlike alpha and beta radiation, which are ejected subatomic particles, gamma rays are part of the electromagnetic spectrum. They are high frequency, high energy photons that travel further than particles. If alpha and beta particles are bees, then I guess gamma rays are mosquitos. They’re smaller than bees or wasps, penetrate the deepest (think malaria vs. bee sting), and you need high-density barriers to stop them from getting to you. Lead, concrete, and tungsten can block gamma rays.
No analogy is perfect – just understand that gamma rays will travel further through your body, much like an x-ray.
What is a half life?
A depressing character in a movie who only lives up to half his potential, never really connecting with others or recognizing his own gifts?
Wow, ok, sorry, that went dark fast. But no. Not that kind of half life.
There are two factors to consider when selecting a clinically useful isotope:
The type of radiation it emits.
Is the therapeutic goal to destroy the diseased cells with beta particles? Or are we trying to picture the diseased tissues with gamma ray imaging techniques?
The time it takes to lose half of its energy, or its half-life.
A radioactive isotope will lose half of its energy in a specific time, every time. Different from the pharmacokinetic models of a drug’s half life, we use k =0.693/t1/2, where k represents the decay constant and t represents time.
A brief, fascinating sidebar: this phenomenon is so precise that Pierre Curie, in his 1902 report titled 'On an absolute measure of time,' predicted the utility of radio decay as a standard for defining time. This prediction was realized in 1967, when the International Committee of Weights and Measures redefined the "second" based on the decay of Cesium-133.
Half lives are an important consideration for therapeutic use and patient safety. Is prolonged exposure important to therapeutic goals? Or is a short lived isotope preferred for a quick image? Both have roles.
Calibration of Radiopharmaceuticals
Let’s take a step back and compare nuclear pharmacy to a traditional pharmacy setting. When I go pick up a prescription at my local community pharmacy, it doesn’t really matter what time I get it. Obviously, my pharmacist would love to have an idea of when I’m coming to pick it up so he can make sure it’s ready for me, but the medication will be the same, whether I pick it up today or tomorrow or a week from now.
Because of radioactive decay, time does not allow nuclear pharmacists the same luxury. With nuclear medicine, hospitals have to know when their patients will receive the dose, and nuclear pharmacists have to know how much activity to send to the hospital ahead of time.
If a hospital says they want 30mCi of Tc-99m at 1200 but they don’t use it for another six hours, they will only have 15mCi for that patient. They might not get good results from their scan with a lower activity, and the patient will have to undergo the procedure again. Therefore, hospitals send their prescriptions with calibration times. A calibration time is the time they plan on administering medication to their patient.
Radiopharmaceuticals for Image Diagnostics
Nuclear medicine can be administered for image diagnostics or for therapy. Nuclear imaging typically uses medications labeled with gamma-emitting isotopes (remember, gamma rays travel the furthest through tissue and give us the best picture), which can show shape and function of the targeted organ.
Ideally, imaging agents have short half-lives because a long-lived nuclear response is not necessary and may cause unnecessary harm. Patients are administered the medication, placed in a machine which “takes a picture”, and then they carry on with the rest of their day. With imaging agents, they decay quickly so patients can live their lives and go into government buildings without being accused of carrying nuclear weapons. (Government buildings actually have radiation sensors, so if a patient did go to one of them, even a post office, they could set off alarms. Doctors give out placards with the medicine information on them for this exact reason.)
There are too many nuclear drugs to list all of them, but some of the more common imaging agents and their uses are listed below:
Technetium-based (Tc-99m) (t1/2 = 6 hours)
o Sestamibi (Cardiolite) and Tetrofosmin (Myoview)
Use: cardiac imaging
Sestamibi and tetrofosmin work similarly to visualize patients’ hearts. They are most often used in stress tests to illuminate heart muscle when a patient is relaxed and when a patient’s heart is under stress. This helps physicians determine risk for heart attacks by showing diseased areas of the heart.
Images created from a heart scan following injection with sestamibi. The brighter colors show the most activity, while darker areas are parts of the heart that are not as engaged. (Image)
o Pyrophosphate (PYP)
Use: cardiac imaging, bone imaging
Since pyrophosphate gets taken up by bones and by the heart, it works to image both systems. Unlike sestamibi and tetrofosmin, it does not give information on future heart attacks. Pyrophosphate is typically used to confirm a diagnosis of myocardial infarction. In the skeletal system, it shows areas of abnormal bone growth.
o Medronate (MDP)
Use: bone imaging
Medronate works in the skeletal system to show areas of abnormal bone growth. Unlike pyrophosphate, it only works here. Some nuclear pharmacies will dispense medronate to image bones in horses, so they can see bone fractures leading to lameness.
Images created from a bone scan following medronate injection. The darker areas show locations of bone metastases from a renal tumor. (Image)
o Mebrofenin (Choletech)
Use: liver imaging, gallbladder imaging
o Macro Aggregated Albumin (MAA)
Use: lung imaging
Macro aggregated albumin is an injectable medication for viewing pulmonary perfusion. It shows physicians where shunts should be placed, as well as helps determine the presence of blood clots in the lungs.
Images of a lung perfusion test using macro aggregated albumin. The right lung in the perfusion study is not visualized due to a pulmonary embolism stopping medication from reaching the lung tissue. (Image)
o Mertiatide (Mag3)
Use: kidney imaging
Mertiatide helps physicians diagnose kidney abnormalities, as well as kidney failure. It also shows kidney stones or other obstructions in the urinary tract. It can be used for both kids and adults.
o Tilmanocept (Lymphoseek)
Use: lymph node imaging
By creating a map of the patient’s lymphatic system, tilmanocept helps physicians find the lymph node closest to a patient’s primary tumor (the first tumor growth site). Right now, this study is mainly used for patients with breast cancer or melanoma.
Image produced in a patient with melanoma following tilmanocept injection. The injection is administered near the tumor site (by the elbow of this patient), and the first lymph node from the tumor is identified (near the armpit). (Image)
o Pentetate (DTPA)
Use: kidney imaging, brain imaging
Pentetate works similarly to mertiatide in the kidneys. However, it can also accumulate in the brain. It shows physicians if patients have an abnormal blood-brain barrier or if there is blood flow indicative of tumor growth.
Gallium-based (Ga-68) (t1/2 = 66 minutes)
o Gozetotide (Illuccix)
Use: prostate cancer imaging
Illuccix is used to diagnose prostate cancer with prostate-specific membrane antigen (PSMA) expressing tumors. It is especially helpful for patients with metastatic prostate cancer because it can provide a clear picture of the cancer spread.
Image created following injection with Illuccix. The colorful spots are places of high uptake in the body, likely metastases from prostate cancer. (Image)
o Dotatate (Netspot)
Use: neuroendocrine tumor imaging
Netspot is used to diagnose neuroendocrine tumors that are somatostatin-receptor positive. Neuroendocrine tumors can be present throughout the body, so this medication helps determine how far it spreads and where the tumors are located.
Image created following injection with Netspot. The dark spots are places of high uptake, most likely metastases from the original neuroendocrine tumor. (Image)
Iodine (t1/2 = 13.2 hours)
o Sodium iodide I-123
Use: thyroid imaging
Indium (In-111) (t1/2 = 2.8 days)
o Oxyquinoline (Oxine)
Use: radiolabeling white blood cells
What I imagine a patient looks like after being administered radioactive blood. (Image)
In this situation, In-111 is used to tag white blood cells so they emit gamma waves. In what is probably the coolest pharmaceutical procedure in the business, we take 60 milliliters of blood from the patient and isolate their white blood cells. We then tag the small button of white blood cells with In-111 oxyquinoline and send it to the hospital for readministration to the patient.
After it is readministered, the patient’s inflammatory processes lead the white blood cells to the site of interest. Physicians often use white blood cell studies to locate abscesses or infections. White blood cell studies can also be used with technetium-99m and the drug exametazime (Ceretec).
Therapeutic Radiopharmaceuticals
When using nuclear medicine for therapy, we want medicines that pack a punch. These isotopes are usually alpha or beta emitters, which don’t travel as far as gamma rays, so the radiation effect is more localized. In order to get a therapeutic effect, the target areas must be exposed to radiation longer than they are with purely-diagnostic agents. For that reason, therapies often have longer half-lives than imaging medicines. Between the chosen isotope and the long half-life, we get a localized effect.
High energy radiation disrupts DNA molecules, which is why it is harmful to life. However, if radiation disrupts the DNA of a cancer tumor, it could kill the tumor. WIN.
Unlike radiation therapy (using external machines to administer radiation to a tumor site), nuclear medicine is an internal treatment. It can be administered directly to the site of interest, or it can target specific membrane receptors on diseased tissue. This strategy reduces off-target collateral damage.
With the long half-life of these agents, patients’ lives can be upended by safety precautions (for themselves and others), but the benefit often outweighs the downside. That is, as long as they don’t have to pick up any packages at the post office.
Nuclear medicine therapies are an up-and-coming area, with many new products under investigation right now. Here are a few that already have FDA approval:
Iodine (t1/2 = 8 days)
Use: thyroid cancer treatment, hyperthyroidism treatment
In a healthy patient, the thyroid absorbs iodine from the diet to create hormones. In a patient with thyroid cancer, we can use this same process to introduce radioactive iodine to the thyroid. Once there, I-131 gives off beta particles, which damage cancerous DNA in the thyroid and cause the tumor to shrink.
I-131 is also used for hyperthyroidism, which is fairly common in cats! Many nuclear pharmacies will make doses for veterinarians to treat cats with thyroid problems.
Yttrium (Y-90) (t1/2 = 2.7 days)
o Ibritumomab (Zevalin)
Use: Non-Hodgkin’s lymphoma treatment
O Microspheres (TheraSphere)
Use: liver cancer
TheraSphere is a drug device made from glass microspheres, which is delivered directly to the liver at the site of an inoperable tumor.
Lutetium (Lu-177) (t1/2 = 6.6 days)
o Vipivotide (Pluvicto)
Use: prostate cancer treatment
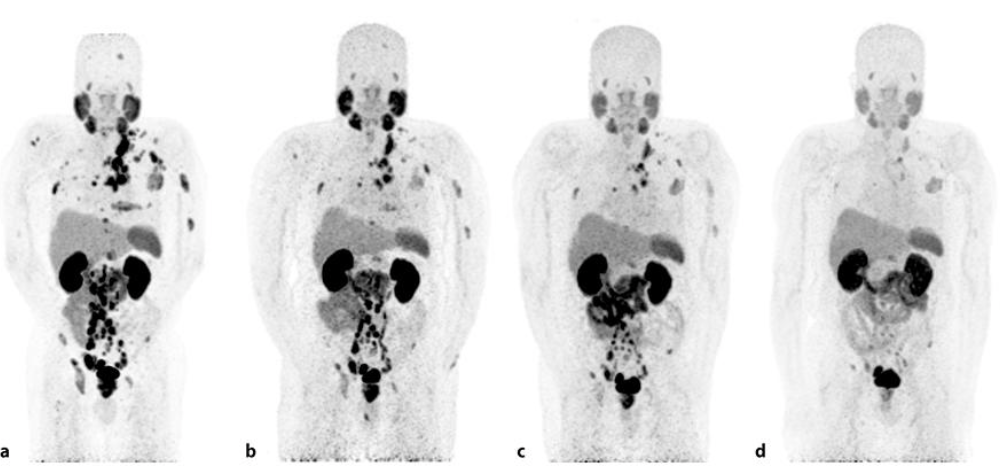
Images produced from a patient with metastatic prostate cancer. The first image is at the beginning of Pluvicto treatment and the last picture is the same patient after six cycles of treatment. The dark spots are tumors (except for the kidneys and bladder, which hold more radiation than other organs since the medicine is flushed from the body via urine). (Image)
o Dotatate (Lutathera)
Use: neuroendocrine tumor treatment
What’s unique about nuclear pharmacy practice?
USP <825> and Lab Specifications
The United States Pharmacopeia (USP) creates regulatory standards that the Board of Pharmacy (BOP) enforces to ensure the quality and safety of medications across healthcare. USP <825> sets standards across radiopharmaceutical use, applying to pharmacies and hospitals. It details things like how to prepare and dispense nuclear products, both sterile and nonsterile. It also references other chapters, like USP <795> and USP <797>, which you hear about in a hospital setting.
Within a nuclear pharmacy, “clean” spaces are defined by various environmental controls, which must be monitored and documented frequently. The International Standards Organization (ISO) details classifications for cleanrooms based on particles suspended in air per cubic meter. The area with the lowest ISO classification is the “cleanest”.
USP <825> details what particle counts are acceptable for performing different tasks. In other words, they tell you what type of ISO classification different areas of the pharmacy need to have. Preparing a sterile radiopharmaceutical takes place in a laminar flow hood - an ISO class 5 environment. The buffer room in which the laminar flow hood exists must be in a minimum of an ISO class 8 environment. If you’re working in a hood, you want everything to be as sterile as possible with as few particles as possible. This is why a “cleaner” environment is needed inside the hood compared to the area where you stand.
Richard Simmons may have been prepared for the sweat of a nuclear pharmacy without A/C, but the rest of us just aren't. (Image)
Along with cleanliness, there are many other considerations when stepping into the restricted areas of a nuclear pharmacy. The temperature and humidity levels must be within a prespecified range. (You’d think this sounds easy thanks to modern technology. Then you work a shift in 100-degree weather when the HVAC system gets clogged with pollen. Now you wish you paid more attention to the blessing of air conditioning.)
Pressure is also incredibly important to document within ISO certified areas. If a room has positive pressure, that means air flows out of the room. If it has negative pressure, that means air flows in. While negative pressure is important to maintain in radioactive drug vials to prevent radiation from leaking out, positive pressure is also important for a room.
Think about the ISO classifications and the particles. You don’t want a lot of particles to flow into an area that has few particles. Therefore, you want your cleaner places to have positive pressure so the air will flow out and into the dirtier places. A disruption in pressure can lead to a compromised lab.
When you look in the fridge and see your milk's gone bad, you know it's time to stay away. (Image)
A compromised lab, whether from temperature or pressure or particles, can have serious outcomes for the medicines. Everything we consume has an expiration date. Think of a jug of milk. If it’s kept in the fridge, you can drink it all week. But if your fridge breaks and warms up, maybe you can drink that milk for an hour (if you feel brave) before you should throw it away. The same principle applies to nuclear medicine.
Expiration dates account for stability, but beyond use dates (BUDs) account for both stability and sterility. A BUD is the time after when a compounded medicine can no longer be given. It depends on the medicine dispensed, as well as the environment it is prepared in. If your lab is too hot because your air conditioner is on the fritz again, your medicines might have a shorter BUD. Just like you wouldn’t want to drink spoiled milk, you wouldn’t want to be administered medicine after its BUD.
The Nuclear Regulatory Commission
We’ve talked a lot about USP <825> and how it relates to pharmacy practice, but its rules would be nothing without an agency to enforce it. This is where the Nuclear Regulatory Commission (NRC) comes in.
The NRC oversees any location that uses nuclear materials, from pharmacies and hospitals to power plants and weapons-developing facilities. They enforce rules on security, waste management, and use of nuclear materials. These are all the rules that USP <825> dreamt up, just mandated by another organization. The NRC will inspect nuclear pharmacies on a regular basis to ensure everyone is following safe and sanitary practices.
Note: this is NOT what an NRC inspection looks like. (Image)
We’ve already established that nuclear medicine takes a lot of coordination between pharmacies and hospitals. To throw an extra wrench in the system, now we have to coordinate practices approved by USP <825> and the NRC.
Important Equipment for Nuclear Pharmacies
USP <825> and the NRC require personal protection when using radioactivity, so nuclear pharmacies need a lot of specialized equipment. Every syringe is placed in a shield with a lead-lined glass window, so you can see the liquid drawn up while still protecting your hand. Every vial is placed in a shield with a magnetized cover to protect the user from every angle, including the septum of the vial. There are lead-lined glass shields in the middle of the hoods to protect the abdomen from radiation exposure. These, among others, are some of the precautions taken to protect employees in a nuclear pharmacy. This equipment decreases radiation exposure to personnel.
Since radiation exposure is such a big concern when working in a nuclear pharmacy, it is important to verify how much radioactivity is present. We can’t “see” radioactivity; a vial of saline looks exactly like a vial of sodium pertechnetate, even though one of them is highly radioactive. Therefore, we need special tools to measure radioactivity.
The well on the left reads radioactivity. The screen on the right tells the viewer the amount of radioactivity present in the source. (Image)
A dose calibrator measures activity based on isotope. You select the isotope you’re working with and place your source (usually a syringe or a vial) in a well. The well protects you from radiation while it reads how much is given off by the source. There is so much more we could say about dose calibrators, but the deep science of dose calibrators is above my pay grade. The important takeaway is that they measure radioactivity and verify that you give the correct dose to the patient.
While a dose calibrator works based on a known isotope, a multichannel analyzer (MCA) can help identify an unknown isotope. When you put a sample in the machine, it filters the different types of activity given off. Based on the activities it reads, you can determine what your isotope is.
One of the most important tools in a nuclear setting is a survey meter. You may know it as a Geiger counter. It has a probe, a handle with a flat circle like a pancake at the end, and is attached to a box. You wave the pancake over an area where you suspect radioactivity, and it chirps when it detects radiation. There is always background radiation no matter where you are, but more frequent chirps indicate more radiation in the area.
This survey meter has a pancake probe. (Image)
We use survey meters in the lab to see if our gloves are contaminated. We use them in the dispatch area to make sure all our boxes are free of radioactivity before we send them out. We also use them everywhere in the office, from the clean room to the breakroom, to make sure there is no contamination on any surfaces. If you aren’t careful, it can be easy to step in radioactivity and track it around the office on the bottom of your shoe. Survey meters make sure radioactivity is only where we expect it to be.
Transportation of Radiopharmaceuticals
(Image)
Nuclear pharmacies are not located in hospitals. Therefore, transportation time is an important consideration. You don’t want to order pizza and have it arrive cold. If you’re a cardiologist ordering a sestamibi for a cardiac stress test, you don’t want it to arrive without enough radioactivity. Just as if you owned a pizza shop and wanted to make sure your pizzas left at the right time, that’s the goal in a nuclear pharmacy. Only, these pizzas are radioactive.
When a pharmacist or technician fills a prescription, the dispatch team packages it according to the hospital or clinic. If the wrong prescription ends up in the wrong box, it cannot be delivered to the right place later (because of the cold-pizza rule just discussed). Therefore, it is extremely important to get all the doses sorted to the correct place.
There are systems to prevent errors, of course, which helps the pharmacy flow smoothly. The dispatch team are experts in knowing hospital distances, so they have the timing down for when to leave for each route. Multiple hospitals receive deliveries from the same driver, so a driver does not have to keep returning to the pharmacy between each drop-off. It helps things stay organized in the pharmacy and in the hospital so everyone can be happy.
Nuclear pharmacy is all about adapting. It is a quickly expanding field, with new uses being studied every day. Even within a nuclear pharmacy, you must be prepared for anything. A hospital can call at any moment and ask for a dose to arrive ASAP. Do you have to make that vial? How long will it take to scrub in to the clean room and draw the dose? How many drivers do you have available? How far away is the hospital? Will there be traffic?
All these considerations, and more, go towards being a nuclear pharmacist. If the state announces that a major bridge is shutting down and your primary client just so happens to be on the other side of the bay (it happens), you need to know how to adapt and get the prescriptions there, regardless of anything the world may throw at you.
Waste Management for Radiopharmaceuticals
And you thought your hazardous waste was hard to keep straight. Level up, yo. (Image)
One of the biggest curveballs in pharmacy, no matter where you practice, is waste. Your goal is to protect and treat patients, but you don’t want to create too much waste. Nuclear pharmacies must discard unused medications, as you would in a community pharmacy, as well as dealing with needles like you might have in a hospital pharmacy.
However, we have the additional concern of radioactive waste. Just a smidge problematic, no?
In a nuclear pharmacy, we separate waste by isotopes. Very-short-lived isotopes (like gallium and its half-life of 66 minutes) will be disposed of in one lead-lined trash can. Short-lived isotopes (like technetium and its half-life of 6 hours) will be disposed of in a different lead-lined trash can. Longer-lived isotopes have their own lead-lined trash cans, too.
The radioactive materials sit to decay in these lead-lined trash cans. Once there is minimal detectable radiation, they can get sent away to a nuclear waste site. However, the truck drivers will not accept trash if it has detectable radioactivity. (This is where our survey meters come in handy!)
Hours of Operation in a Nuclear Pharmacy
What deters people from pursuing a career in nuclear (even more so than radiation exposure) are the hours of operation. Since hospitals have calibration times starting as early as 0700 and we have to factor in half-life and transportation time, we start shifts as early as midnight. We process prescriptions in intervals known as “runs” and complete several runs in a day.
First run occurs in the middle of the night. Pharmacists arrive at their pharmacies at midnight and have their doses out the door by 0400. With the help of technicians and a dispatch team, and depending on the size of the facility and the demand, they can handle almost 1,000 doses during first run.
A pharmacist during on-call hours. (Image)
Second run starts a few hours after first run ends, depending on the pharmacy. This one has fewer doses, since most doses go out during first run. Pharmacies may have a third run and fourth run later in the day, but afternoons usually are for as-needed doses that were not predicted earlier. A hospital or clinic can call their nuclear pharmacy and ask for a dose to be sent at a specific time later in the day, sometimes as soon as possible.
Although waking up before sunrise can be tough, it pays off on the other end. Pharmacy doors close before 1700. From the time doors close for the night (or mid-afternoon) until the time doors open to start a new day, a pharmacist is on-call. If a hospital or clinic urgently needs a dose, their request is routed to the on-call pharmacist, who comes back in to prepare it. There is also usually an on-call driver for these situations.
However, thanks to the unusual hours of the nuclear pharmacy, sometimes a requested dose comes soon before the next shift is meant to start. In that case, the on-call pharmacist might pull out a cot and take a nap in the pharmacy before getting up to prepare first run all over again.
What training is required to become a nuclear pharmacist?
Now that you’ve heard all about nuclear medicine and the excitement that comes with working in a nuclear pharmacy, you’re probably saying to yourself, “Wow, this is amazing! I can’t wait to be a nuclear pharmacist!” I get it – I clearly get excited about radioactive drugs, too. However, there are a couple more things to know about this amazing field before you jump into it.
Homer as an Authorized User? Be afraid...be very afraid... (Image)
A lot of training goes into being a pharmacist in a nuclear pharmacy (which is probably surprising to no one, since I know I wouldn’t want someone handling radioactivity without a lot of training). First, you have to complete a PharmD degree at an accredited school. Then, you have to become an Authorized User and may choose to complete a board certification in the nuclear pharmacy specialty.
An Authorized User is someone who is listed on a radioactive materials license, either in a pharmacy or a hospital. Authorized User training consists of two parts: classroom learning and on-site learning. There are several ways to obtain classroom learning, either through recognized programs at universities or through nuclear pharmacy companies. Some universities have programs through their colleges of pharmacy: University of Arkansas, University of New Mexico, University of Oklahoma, University of Tennessee, and Purdue University. There are also certificate programs offered through most of the aforementioned universities. These programs teach students about radiation safety and radiation principles in a much more in-depth analysis than discussed in this post.
On-site learning is supervised by an Authorized User. There are a lot of tasks to cover, from sending and receiving radioactive packages to preparing equipment to preparing patient doses. A student in pharmacy school can get intern hours by working at a nuclear pharmacy while they’re in school. When they graduate, they already have a lot of on-site training under their belt.
Pharmacy schools may also offer APPE rotations in nuclear pharmacies, which can help cover the required hours, too. However, if someone did not have the opportunity to complete training before hire, many nuclear pharmacy companies will provide this training for new hires or will help pay for their employees to receive it. To become an Authorized User, someone has to complete 200 hours of classroom learning and 500 hours of on-site training.
Along with getting the Authorized User certification, you may also receive certification from the Board of Pharmacy Specialities (BPS). Nuclear pharmacy is a recognized specialty within the world of pharmacy, although certification is not a requirement to become a nuclear pharmacist. This is the Nuclear Pharmacy Specialty Certification (BCNP). The BPS website has more information on the exact parameters to earning this certification and reaching the final step to becoming a full-fledged nuclear pharmacist.
The tl;dr of Nuclear Pharmacy
Nuclear pharmacy is a very niche field within the pharmacy profession. It has a lot of idiosyncrasies that make it unique and challenging. There is a lot of work that goes into becoming a nuclear pharmacist, and even then the work doesn’t stop. Despite the odd hours and radiation exposure, hundreds of people work in this small field.
If you found yourself interested in this post, nuclear pharmacy could be a career for you. Just because it is different and unknown to you doesn’t mean you can’t excel. Everything is new to everyone at some point, and even the masters of this profession were once beginners wondering how radiation science worked. Use this post as a starting point and do your own research –
Could nuclear pharmacy be right for you?