A Pharmacist’s Primer on Antimicrobial Resistance: Part 1
Steph’s Note: This week, we have the return of infectious diseases. And not just any old disease state review (which we also love, don’t get me wrong), but the start of a beast of a topic: antimicrobial resistance. If you’re like me when I was a student, my head would start spinning (and I would mentally check out from being overwhelmed) whenever the discussion turned to resistance. Shoot, I was struggling to learn the basics of bugs and drugs, and then my professor had the nerve to throw that out the window and tell me a drug wouldn’t work for that bug I just learned!? GASP! Don’t worry, there’s hope. You WILL learn this stuff.
And here to help is Anthony Cruz, a P4 at the VCU School of Pharmacy. He is very interested in antibiotics and microbes after earning a degree in Biochemistry and Molecular Biology from Oklahoma State. ID first piqued his interest when working in a pathogenic microbes lab, plating and identifying a variety of bacteria, and he chose a career in pharmacy hoping to learn more about infectious diseases from a medical standpoint. He hopes to complete a residency and practice as an infectious diseases pharmacist, much like his mentor for this post, Dev Chatterji (of previous tl;dr fame).
Antimicrobial resistance (AMR) is a global public health threat that is concerning worldwide. It’s also one of the core areas that organizations such as the CDC and WHO are trying to combat via antibiotic stewardship efforts. While there are several factors that cause AMR, overuse and suboptimal use of antibiotics are modifiable factors that pharmacists can play a pivotal role in. Several organisms are more likely than others to develop or acquire resistance.
(Image)
This series of posts will focus on general principles of AMR and then summarize some of the guidance we have on managing the more looming “threats" that the CDC and other organizations have identified. First and foremost, we are going to start with the basics…
Disease Burden Associated with Antimicrobial Resistance
Like all good stories, first we must begin by establishing some stakes. A recent 2024 publication in The Lancet provided a systematic analysis of the disease burden of AMR. It found that, in 2021, bacterial AMR contributed to an estimated 4.71 million deaths, while 1.14 million were directly due to resistant infections. Fortunately, in recent years we have been able to halve these numbers in children younger than 5; however, our rates in adults over 70 years old have skyrocketed by over 80%!!
Although these rates vary globally, 2.8 million resistant infections occur each year in the United States alone, according to the CDC’s 2019 Antibiotic Threats Report. However, thanks to the efforts and improvements in US healthcare, we have been able to lower our number of deaths attributable to AMR by over 15% since the 1990s! Woot! The downside? According to the CDC’s 2022 Special Report, that progress was damaged by COVID-19’s significant impact on our health systems!
However you look at it, the bottom line is that our current efforts are not outpacing the expanding burden of disease. In 2050, an anticipated 8.22 million (6.85-9.65) deaths associated with AMR could occur globally. This insane number and exponential increase is largely attributed to our southern neighbors in the western hemisphere, as well as a smaller portion from South Asia.
Now before we get all doom and gloom… Fortunately, it’s also estimated that 11.1 million deaths could be averted if our efforts continue to be successful in managing these pesky bugs.
So, have I convinced you of the importance of learning about AMR and antimicrobial stewardship?
Back to the Basics of Antimicrobial Resistance
AMR is an extremely broad topic, as microbes exist as a very broad range of organisms. There is a wide variety of bacterial species to consider, and each has its own unique traits that may be difficult to overcome. Not to mention, we have to remember there are more bugs than bacteria to worry about! Don’t forget the other microbes like fungi, parasites, atypicals, and even protists to consider.
For this series of articles, we will focus on antibacterial resistance specifically and expand on the different ways bacteria we commonly encounter in the clinical setting can develop resistance to our various choices of drugs.
What resistant bacteria would look like busting out their weapons, if this was Star Wars. (Image)
To keep it simple, AMR occurs when there is decreased ability of an antimicrobial to kill or inhibit the growth of a microbial organism. We recognize this in practice via antimicrobial susceptibility testing. Recall from this post on bacterial antimicrobial susceptibility testing, we utilize quantitative in vitro testing systems to measure drug activity against specific bacteria via the MIC (minimum inhibitory concentration).
An individual drug’s MIC is then compared to an established breakpoint [recall that breakpoints are established by groups such as Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST)]. A bacterial strain reported as susceptible to that individual drug will have a MIC value at or below the breakpoint—this indicates a high likelihood of therapeutic success. A bacterial strain reported as resistant to that individual drug will have a MIC value above the breakpoint—meaning a high likelihood of therapeutic failure. So now the question in this post is why or how is that bacterial strain resistant?
In any bacterial species, resistance to antibiotics must have a point of origin. They haven’t always been resistant. Much like us, bacteria are living organisms and have a fairly diverse immune system. We can think of an individual bacteria’s resistance as similar to our own immune system: innate or intrinsic resistance OR adaptive or acquired resistance.
Intrinsic Resistance: This refers to the inherent properties of a bacterial species that result in lack of response to an antibiotic or class of antibiotics. While our human innate immunity features include things like our tough and dry skin, mucous membranes, and proteins, bacteria have similar intrinsic factors that allow them immediate resistance to certain types of antibiotics (e.g., cell walls, drug binding proteins, etc.)
Acquired Resistance: This refers to the emergence of resistance due to a change in phenotypic characteristics of a bacterial species. Much like how our immune system will adapt and respond in a directed and targeted manner to a pathogen, certain species of bacteria also adapt and can suddenly confer resistance to an antibiotic they were formerly susceptible to.
Just go ahead and take a mental pic of this. (Image)
Mechanisms of Antimicrobial Intrinsic Resistance
(Image)
Intrinsic bacterial factors begin with relatively simple mechanics, starting with basic classification of bacteria: gram positive and gram negative. As you may recall, gram negative bacterial cell walls have that double layer cell membrane, as well as a much thinner peptidoglycan layer that differentiates them from our gram positives. One of the best examples of intrinsic AMR is resistance of gram-negative organisms to the glycopeptides (e.g., vancomycin). These drugs are unable to penetrate gram negative organisms’ outer membrane to reach the target.
Another intrinsic factor that also inhibits drug permeability is the biofilm. Even though individual bacteria may be weak, together they can form something like a Roman tortoise formation. If you’re not familiar with this Roman legion tactic, the bacteria organize themselves into a structure that prevents the human immune system and antibiotics from being able to effectively target these colonies. Biofilms are common among both gram positive and negative bacteria.
Cool…but not cool, right?
Up to 65% of infections are associated with biofilm formation, and 80% of chronic infections are due to these large, difficult to break up colonies. Fortunately, this defense mechanism can frequently be overcome by standard therapies.
Lastly, porins and efflux pumps offer methods for bacteria to clear out drugs that do make it inside, or ones that target other components of the cell. Porins are proteins that generally allow the otherwise relatively impermeable cell wall barrier to become permeable to water and nutrients. BUT certain environmental pressures can result in controlled expression or increased specificity of those porins, which prevents many antibiotics from entering the cell. This is the case for Pseudomonas aeruginosa.
In the event that antibiotics do enter the cell, efflux pumps are capable of pumping out antibiotics before they are able to cause any damage. Normally, efflux pumps serve to regulate the bacterial environment, biofilm formation, and many virulence factors like toxins. This is generally the most common mechanism of resistance across many types of bacteria. However, the most famous of these species are Pseudomonas and Acinetobacter since they have well-expressed and very potent efflux pumps. These efflux pumps can rule out entire classes of antibiotics in the case of Acinetobacter and a very wide range of antibiotics in the case of Pseudomonas.
Mechanisms of Antimicrobial Acquired Resistance
The major focus for this series of posts will be on acquired resistance. Bacteria grow much faster than we do, and therefore, they also evolve much more rapidly. Each successive generation of bacteria growing during infections has an opportunity to mutate a resistance to certain antibiotics. While these genes can be identified with certain tests, these tests are time consuming. They also frequently result in severe limitations in our choice of treatment.
(Image)
Simple adaptive resistance starts with the previously discussed innate and intrinsic resistance mechanisms being expressed more in response to antibiotic exposure. Work smarter, not harder, right? So bacteria beef up the defenses they already have first. The genes that encode for these features can be activated rapidly within existing bacteria. They can then be horizontally transferred through plasmids, as well as vertically transferred to subsequent generations following conjugation. As a result, certain innate features may be more rapidly spread during the course of the infection, resulting in a longer period of symptoms.
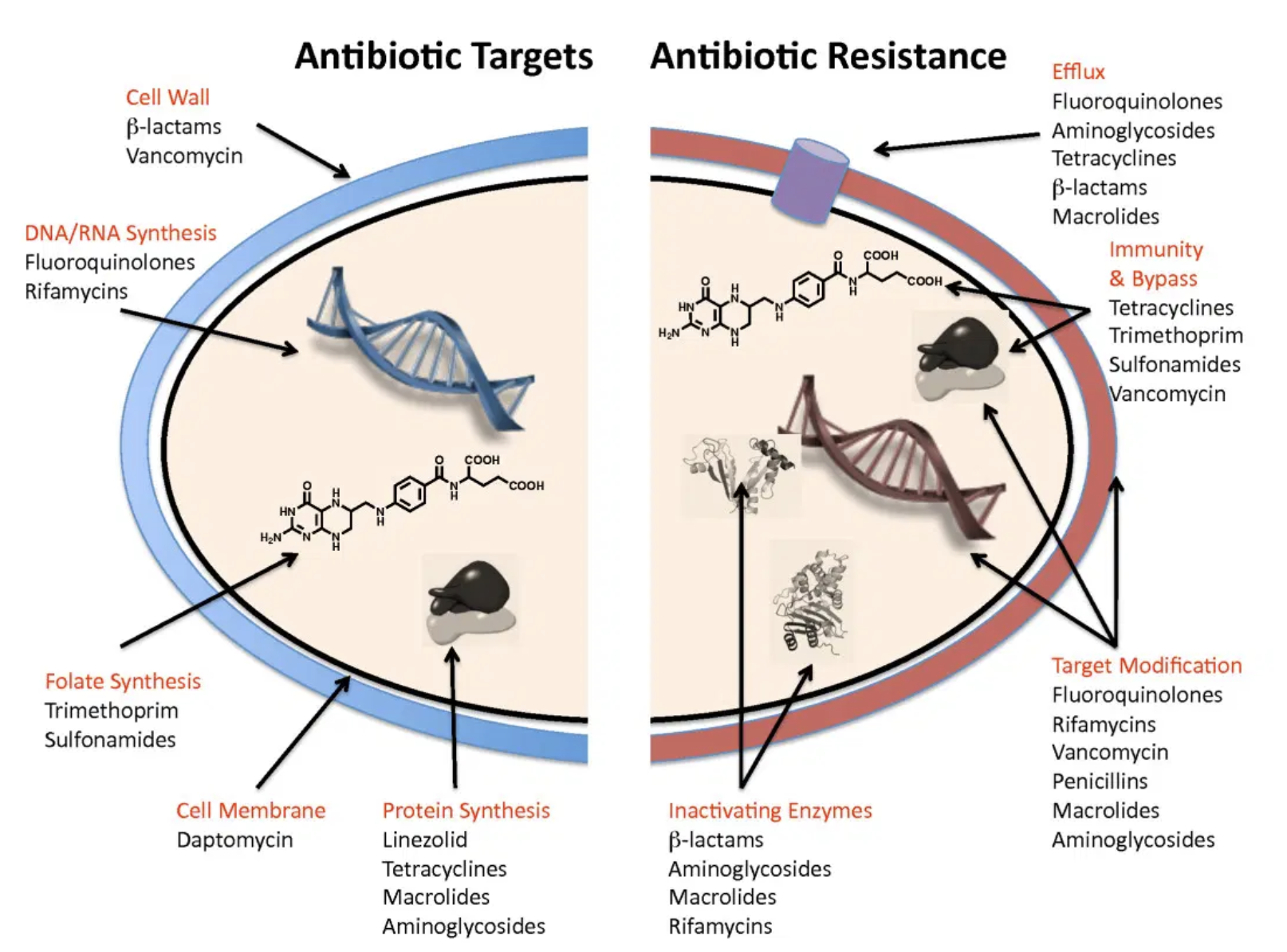
Other mechanisms by which resistance occurs are better summarized into four basic mechanisms for explanation purposes (see chart below). More details will be discussed in subsequent posts when talking about specific types of bacteria species.
The tl;dr of This Introduction to Antimicrobial Resistance
(Image)
Overall, there is a wide variety of mechanisms to consider for how a bug and a drug might mismatch, and there is an equally wide range of ways these mechanisms can be transferred. Horizontal and vertical transfers of these genes and features result in this being an ever-growing problem. Each infected patient may require a very different treatment answer than the next as new enzymes or genetic drift will always occur, and thus the work will always be present to research and identify these mechanisms.
Unfortunately, because of the complexity of resistance, there is no one immediate solution to our problem. Fortunately, we have had several decades of successful antibiotic development since the invention of penicillin, and while at the moment we are arguably winning the ‘antibiotic arms race’, I’m not certain our antibiotic development pipeline will keep up with newly emerging multi-drug-resistant (MDR) organisms in coming years.
Hopefully, the severity of the task at hand should be more apparent compared to when you first started reading this post…more to come!!