What Every Pharmacist Should Know about HIV Pre-Exposure Prophylaxis (PrEP)
Here’s looking at you, HIV PrEP! (Image)
Joe’s Note: Alright, folks. This is it. This is the last piece of the HIV puzzle for us here at tl;dr pharmacy. We really have hit HIV with everything we’ve got. We have HIV cheat sheets, posts discussing the pathophysiology of HIV, articles describing every single antiretroviral drug class, and we’ve even talked about post-exposure prophylaxis (PEP). So this is really it. The final HIV boss: pre-exposure prophylaxis (PrEP).
In my opinion, this is one of the least talked about topics of HIV. Sure, understanding the pathophysiology and treatment is cool. But you know what’s even cooler than treating HIV? Preventing it. In this post, we'll review PrEP pretty extensively. We’ll go over what PrEP is, how it works, who should take it, clinical pearls, and much more. We have quite a lot to discuss today. So let’s get started!
What is HIV PrEP, and who should take it?
As the name clearly suggests, HIV pre-exposure prophylaxis is a combination of medications taken by HIV-negative individuals to prevent HIV infection. So, who should take it?
Per the CDC, PrEP therapy may benefit individuals who test negative for HIV and have ANY of the following:
Have had anal or vaginal sex in the past 6 months, AND:
Have a sexual partner with HIV (especially if the partner has an unknown or detectable viral load), OR
Have not consistently used a condom, OR
Have been diagnosed with a sexually transmitted infection in the past 6 months.
Inject drugs, AND:
Have an injection partner with HIV, OR
Share needles, syringes, or other injection equipment.
Have been prescribed HIV post-exposure prophylaxis (PEP), AND:
Report continued risky behavior, OR
Have used multiple courses of PEP.
I want to start PrEP, so now what?
Let’s say you or your patient meet the criteria above and are interested in starting PrEP. What’s the next step? First thing is to complete a baseline assessment. This assessment should include tests such as:
HIV Test
Must be HIV negative prior to starting PrEP therapy.
Sexually Transmitted Infection (STI) screening including chlamydia, gonorrhea, and syphilis
Patients at risk for HIV transmission are also at risk for other sexually transmitted infections. STI testing should be completed and all positive results should be treated.
Hepatitis B and C screening
Patients at risk for HIV transmission are also at risk for hepatitis B & C. Screening and testing for hepatitis should be done accordingly.
Basic Metabolic Panel
Some PrEP therapy is renally excreted. A baseline serum creatinine should be collected prior to initiation of therapy to assess creatinine clearance and adjust dosing as needed.
Pregnancy Test
Certain PrEP therapy is teratogenic and should be avoided in pregnant patients.
If all the above tests come back clean and the patient is at high-risk for HIV, then PrEP can be started.
What are my PrEP options?
Currently, the U.S. Food and Drug Administration (FDA) has approved three medications for HIV pre-exposure prophylaxis. Two are available in a daily oral pill form, while the other is available as a long-acting injectable form. What are these anti-HIV drugs, and which one should you recommend? Let’s review.
The oral options approved for daily PrEP use include:
Truvada (emtricitabine/tenofovir disoproxil fumarate)
Descovy (emtricitabine/tenofovir alafenamide)
The long-acting injectable form of PrEP approved by the FDA:
Apretude (cabotegravir)
Alright we have a lot to discuss. First, let’s review how these drugs work.
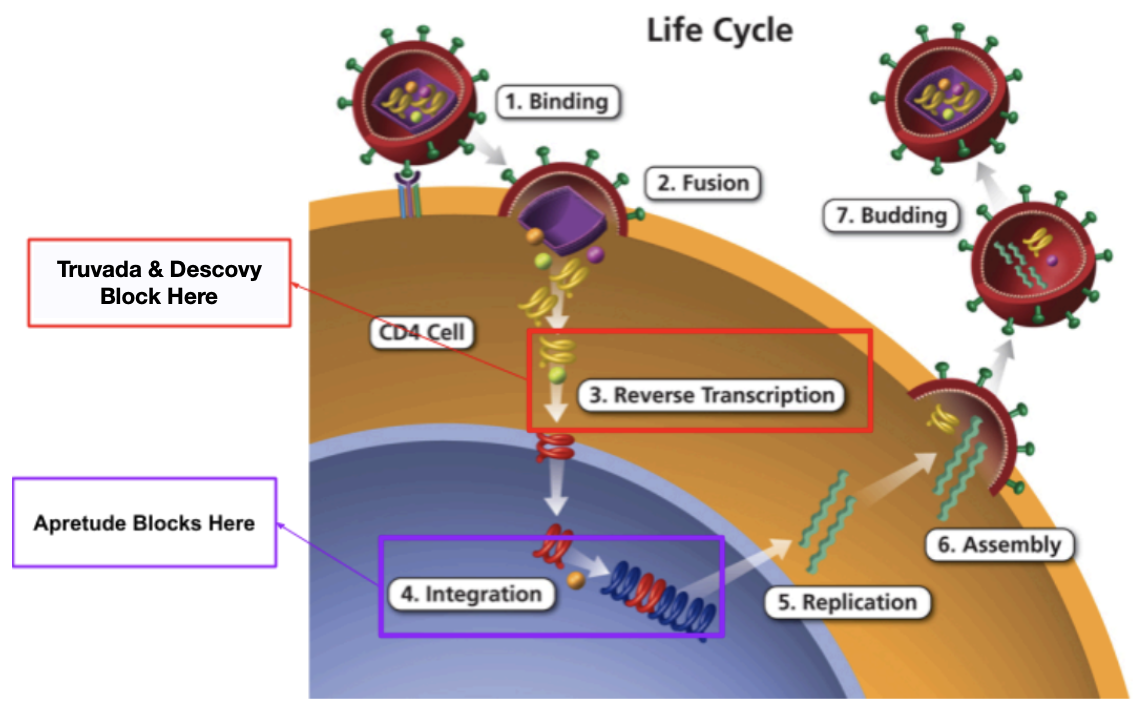
Remember the seven steps of the HIV replication cycle? It goes over how the virus attacks host cells and replicates. If you don’t remember, here is a refresher:
Binding: HIV attaches to a CD4 receptor on the surface of a host cell, typically CD4 T lymphocyte.
Fusion: The HIV virus merges with the host cell, releasing its genetic material (RNA) into the cell.
Reverse Transcription: HIV’s reverse transcriptase enzyme converts the viral RNA into HIV DNA.
Integration: The HIV DNA is inserted into the host cell’s genome using the integrase enzyme.
Replication: The host cell’s machinery produces new HIV proteins and RNA molecule.
Assembly: The viral proteins and RNA come together to form immature HIV virions.
Budding: The immature virions are released from the host cell and mature into infectious HIV particles.
So to treat HIV and/or prevent transmission, we need to block the HIV replication cycle at any of the above steps. However, not all steps of the replication cycle are made equally. Blocking certain steps has been shown to be more efficacious than others. So where do the PrEP drugs work?
Truvada is a combination medication of both emtricitabine and tenofovir disoproxil fumarate. Both of these medications belong to the nucleoside reverse transcriptase inhibitor (NRTI) drug class and work by inhibiting reverse transcription (step 3 of the HIV replication cycle). Specifically, these drugs interfere with HIV viral RNA dependent DNA polymerase, resulting in inhibition of viral replication.
Sheldon is also glad - because it’s pharmaCOOL. (Image)
On the other hand, Descovy is a very similar combination medication that contains emtricitabine and tenofovir alafenamide. Just like Truvada, both of these medications also belong to the NRTI drug class and work by inhibiting the reverse transcription phase of HIV replication (step 3). Just like Truvada, Descovy also has emtricitabine and tenofovir. However, Descovy contains tenofovir alafenamide while Truvada contains tenofovir disoproxil fumarate.
So what’s the difference? I’m so glad you asked.
Long story short, tenofovir alafenamide (TAF) is the active prodrug while tenofovir disoproxil fumarate is the inactive form. Why does this matter? Because tenofovir is not the nicest drug. It has a lot of systemic toxicities including nephrotoxicity, mitochondrial dysfunction, hepatotoxicity, and bone loss. So how did science combat these adverse effects? By creating TAF.
Like we said earlier, TAF is the active prodrug. It was specifically formulated to deliver the active metabolite to target cells more efficiently than TDF at a lower dose, thereby reducing systemic exposure. So yes, TAF is generally preferred over TDF as it has equal efficacy but reduced risk for kidney and bone toxicity compared to TDF.
Alright, now let’s talk about that long-acting injectable form. Unlike our oral options, Apretude (cabotegravir) belongs to the integrase inhibitor drug class. Specifically, it inhibits HIV integrase by binding to the integrase active site and blocking the strand transfer step of retroviral DNA integration (step 4 of the HIV replication cycle). If you’re a visual learner like me, here is a picture that shows where these PrEP drugs work in the HIV replication cycle:
(Image)
Which PrEP therapy should I pick?
So yes, there are three FDA-approved options for PrEP. We went over how they work above. But clinically, which should you use?
Luckily, the FDA makes it very easy to decide as they give pretty clear instructions:
Truvada (emtricitabine/tenofovir disoproxil fumarate): for all patients at risk for HIV through sex or injection drug use.
Descovy (emtricitabine/tenofovir alafenamide): for sexually active men at risk of getting HIV. Descovy is NOT recommended for women who are at risk for HIV through receptive vaginal sex. Descovy is NOT recommended for all patients at risk for HIV through injection drug use.
Apretude (cabotegravir): for all patients at risk for HIV through sex who weigh at least 77 pounds (35 kg). Apretude is NOT recommended for patient at risk for HIV through injection drug use.
So long story short, if your patient is a:
Male at risk for HIV through sex: recommend Truvada or Descovy or Apretude (as long as ≥77 lbs)
Male at risk for HIV through injection drug use: recommend Truvada
Female at risk for HIV through sex: recommend Truvada or Apretude (as long as ≥77 lbs)
Female at risk for HIV through injection drug use: recommend Truvada
The above criteria definitely helps narrow your options. But there are other clinical pearls that should be taken into account when making your decision. Let’s go through them one by one:
I started oral PrEP therapy. How should I take it?
When it comes to the oral formulations, this part can get pretty darn confusing. There are 3 main methods in which oral PrEP therapy could be taken. This includes daily PrEP, on-demand PrEP, and periodic PrEP. Let’s review them all.
Daily PrEP is the most straightforward. As the name suggests, patients partaking in this regimen should take a dose every day for lasting protection. This regimen can suit a lot of people since it provides constant protection from HIV without having to anticipate when the next sexual encounter may be. Starting the daily PrEP schedule may slightly differ depending on how the patient identifies.
Now let’s say for one reason or another, the patient wants to stop oral PrEP therapy altogether. What is the correct way to stop?
Now on to on-demand PrEP. Currently, on-demand PrEP is only recommended for cis men who have sex with other men, as there is NOT enough research to support its efficacy in other groups. On-demand PrEP may suit cis men who infrequently have sex and are concerned about the side effects associated with taking daily medication. For on-demand PrEP, the timing for doses is extremely important.
To take PrEP on-demand, use the 2-1-1 dosing schedule:
Take 2 pills (double dose) between 2-24 hours before sex, then
Take 1 pill 24 hours after the double dose, then
Take 1 pill another 24 hours after that.
If you continue to have sex, then keep taking 1 pill daily until two days after the last intercourse.
Finally, on to the last option for PrEP, which is periodic PrEP. If you don’t want to take daily PrEp forever and on-demand PrEP may not be enough given your intercourse frequency, then periodic PrEP may fit you best. With periodic PrEP, patients should take one pill once a day over a period of time when they want to protect themselves from HIV. That period can be as short or as long as they need it to be. It really depends on their lifestyle. If this regimen fits best, here is the appropriate way to start:
The daily tablet can be continued for however long HIV protection is needed. For example, let’s say that period was deemed as one month. Once the month is up and the patient wants to cycle off PrEP, here is the appropriate way to stop:
As you can see, the periodic PrEP regimen is very similar to the daily PrEP regimen. It’s just a shorter duration that will need “reloading” once therapy is stopped and started again.
What about the injectable PrEP?
Yes, like we discussed earlier, injectable PrEP is an option too and can be considered for specific patients. However, it is important to remember that Apretude is ONLY recommended for PrEP in patients at risk for HIV through sex. It is NOT recommended for use in patients at risk for HIV through injection drug use. That being said, there are a few clinical pearls with this drug. For example,
Apretude can ONLY be used in patients who weigh at least 35 kg (77 lbs). To start Apretude, dosing is as follows:
Initiation injection: Apretude 600 mg intramuscularly once monthly for 2 doses
Continuation injections: Apretude 600 mg intramuscularly once every 2 months, starting 2 months after the last initiation injection
Oh and one other clinical pearl regarding Apretude. If Apretude injections are discontinued, the gradually declining levels of cabotegravir raise the risk of developing a drug-resistant strain if HIV infection occurs at that time. Therefore, it is VERY important that patients taking Apretude keep their regular injection appointments and avoid missing doses.
How effective is PrEP?
Alright let’s talk about it. Generally speaking, HIV.gov estimates that PrEP reduces the risk of getting HIV from sex by about 99% when taken as prescribed. However that number dips to 74% among people at risk for HIV through injection drug use. Again, efficacy is highly dependent on medication adherence. The more adherent, the better the efficacy and outcomes. The less adherent, the higher chance of contracting HIV.
What? You mean real-world medication adherence is less than in clinical trials? Yeah, Michael wasn’t that surprised either. (Image)
Unfortunately, much like post-exposure prophylaxis, there are very few observational studies to assess PrEP efficacy. But there are still some. The first we should discuss is this study that was published by Hugo, et al. This study found that PrEP effectiveness appears to be lower in real-world conditions than is reported in clinical trials. Specifically, PrEP was shown to be effective in 60% of patients overall. As you can assume, efficacy was higher (up to 93%) in patients who had a high amount of PrEP consumption. They also found that PrEP effectiveness was significantly reduced in people younger than 30 years and in those who were socioeconomically deprived. As you can guess, both of these groups showed low amounts of PrEP consumption. So the decreased efficacy was likely NOT drug related but more so secondary to non-adherence.
Not to beat a dead horse regarding adherence and efficacy. But a pharmacokinetic study of oral PrEP estimated effectiveness to be 99% if doses are taken 7 days a week, 96% for 4 doses per week, and 76% for 2 doses per week. That’s why optimal benefit is only achieved with high adherence rates.
Alright, that’s it, folks. Are you officially an HIV expert? I really hope so.