What Every Pharmacist Should Know about Drug-Induced (Acquired) Methemoglobinemia
^^Josef, writing about critical care for tl;dr (according to Steph)
Steph’s Note: Because he is just ridiculously productive and chock full of information, Dr. Josef Nissan is back this week to drop some more tl;dr critical care knowledge. (If you haven’t already, definitely check out his previous posts on vasopressors, heparin-induced thrombocytopenia, and acetaminophen toxicity. These have all been posted within the last 3 months - yes, he’s THAT prolific! Making the rest of us look bad, Josef, seriously :D). Carry on, and teach us again!
If you’re looking for a handy guide to carry around with you when you’re covering the ICU/ED, check out our Pocket Guide to Pharmacotherapy Management of Acute Medical Emergencies. It covers methemoglobinemia and a bunch of other essential critical care topics.
You’re probably questioning why I decided to write about a disease-state with a prevalence of less than 2%. I promise there is a method to my madness. Sure, drug-induced (acquired) methemoglobinemia isn’t terribly common, but that’s exactly why I chose to create this post.
As pharmacists, we’re looked at as the medication experts, and for that reason, it’s extremely important that we’re comfortable recognizing which drugs/toxins may induce methemoglobinemia and how we should treat it. Additionally, this is a common NAPLEX and BCPS topic that you will likely be tested on. So get comfortable with it ;).
Let’s start with the basics. To get a better understanding of methemoglobinemia, let’s go back to physiology 101 and review the structure and physiological functions of red blood cells (RBCs).
Each RBC in the human body contains approximately 270 million hemoglobin molecules. Each hemoglobin molecule is composed of four folded polypeptide chains (alpha and beta chains). Each polypeptide chain has a porphyrin heme group attached.
At the center of each of the four heme groups is an atom of iron in the ferrous (Fe2+) state. Oxygen can reversibly bind to Fe2+ and form oxyhemoglobin, which allows for adequate delivery of oxygen to the tissues. To sum it up visually, here is a nice picture that discusses everything I just talked about:
(Image)
Pathophysiology of Drug-Induced Methemoglobinemia
Now that we’ve reviewed the basic structure and function of hemoglobin, let’s talk about methemoglobin. Btw, this is pronounced met-hemoglobin…not meth-hemoglobin (as Steph’s brain used to want to say). Pronunciation matters.
In methemoglobinemia, at the center of each of the four heme groups is one or more iron atoms in the ferric (Fe3+) state. When iron is in the ferric (Fe3+) state, it prevents the binding of oxygen, resulting in inadequate oxygen delivery to the tissues.
In drug-induced (acquired) methemoglobinemia, there is an acceleration of hemoglobin oxidization from the ferrous (Fe2+) to the ferric (Fe3+) state, resulting in tissue hypoxia. The exact mechanism of why this occurs in certain individuals isn’t fully known but is believed to be a consequence of drug ingestion or toxin exposure.
So where does our role as the medication experts come into play? You guessed it! By familiarizing ourselves with which drugs and toxins can induce methemoglobInemia, we can help expedite the diagnosis and begin treatment as soon as possible to help prevent tissue hypoxia, necrosis, and eventual death.
Drugs and Toxins Known to Induce Methemoglobinemia
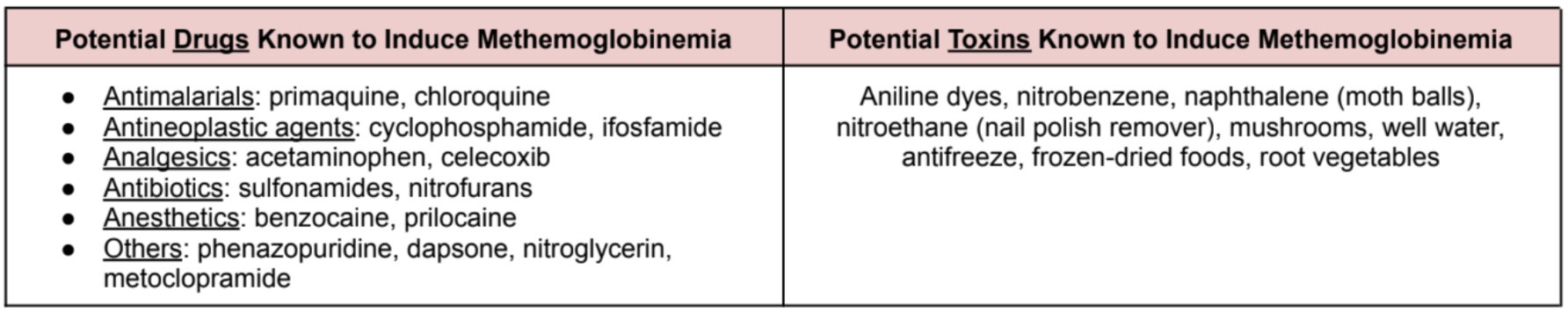
There are a lot of drugs that have been shown to induce methemoglobinemia. Some drugs are commonly used, while others not so much. Here’s a table that I prepared for you that reviews some of the more common drugs and toxins known to cause methemoglobinemia:
Signs and Symptoms of Methemoglobinemia
The severity of the symptoms associated with methemoglobinemia are strongly correlated with the methemoglobin (MetHb) level. The higher the methemoglobin level, the more toxic and life-threatening the disease can be. Let’s review the symptomology below:
MetHb level < 10%: Patients are likely to be asymptomatic but may have a slight alteration of skin color (e.g., pale or blue).
MetHb level 10-30%: Patients may be asymptomatic but typically present with slight confusion and appear cyanotic.
MetHb level 30-50%: Patients typically present with altered mentation, palpitations, headaches, dyspnea, dizziness, and chest pain.
MetHb level 50-70%: Patients present with all the symptoms above plus potentially worsened tachypnea, metabolic acidosis, dysrhythmias, seizures, and coma.
MetHb level > 70%: Levels this high result in severe hypoxemia and death.
Diagnostic Approaches to Methemoglobinemia
As I alluded to earlier, I typically don’t stress too much about the diagnostic methods for many disease-states as this is usually outside of my scope of practice. That being said, this is one of those special diseases where I think pharmacists can play a big role in expediting the diagnosis to help initiate treatment as soon as possible.
Me, everytime my preceptor asked me to complete a med rec
Medication reconciliation. Honest moment… these are my worst nightmare. I never liked doing them as an APPE student, and I still don’t really like them now.
I vividly remember being so excited to start my ED rotation as an APPE student thinking that every second of every day was going to be an adrenaline rush. Little did I know that people don’t code every 2 seconds and that there’s some downtime even in the emergency department.
To make matters worse, whenever there was downtime, guess who had to go and complete a med rec? Yup, you guessed it. Lesson learned, don’t ever believe what the TV medical dramas portray. Healthcare really isn’t always like that…
Anyway, back to methemoglobinemia. Even though medication reconciliations might not be the most exciting thing in the world, they are EXTREMELY important for the diagnosis of drug-induced methemoglobinemia. I cannot stress the importance of collecting a strong patient history and reviewing any potential exposures, including dietary, medications, and/or chemicals known to induce methemoglobinemia.
Outside of medication reconciliation, arterial blood gas with co-oximetry remains the gold standard for the diagnosis of methemoglobinemia. Generally, methemoglobinemia is diagnosed when a MetHb level is 5% or higher. Methemoglobin levels greater than 30% are considered life-threatening and require emergent management.
Other diagnostic approaches include physical examination, complete blood count (CBC), and G6PD testing.
BTW - This is a lot of information. We’ve got a downloadable (and printer-friendly) PDF of this article that you can download here. It’s great for offline viewing and for writing notes.
Medical Management of Methemoglobinemia
Now that we have a better understanding of what methemoglobinemia is, let’s review our treatment options. Below is a table that I made that summarizes the step-wise approach of treating methemoglobinemia:
“Blue Cures Blue” (Methylene Blue)
I bet you haven’t seen methylene blue used too often (or maybe you have, I don’t know). I know I personally haven’t. But methylene blue remains the primary pharmacologic agent used for the treatment of methemoglobinemia.
Does that mean every patient diagnosed with methemoglobinemia requires the administration of methylene blue? Nope. A lot of times supportive care for minimally symptomatic or asymptomatic patients is generally sufficient. Methylene blue should ONLY be initiated if:
MetHb level >30%, OR
MetHb level 20-30% BUT patient is symptomatic and/or has underlying cardiac or pulmonary disease
Mechanism of Action of Methylene Blue
In low concentrations, methylene blue oxidizes NADPH to form leukomethylene blue, which in turn is able to reduce iron from the ferric (Fe3+) to the ferrous (Fe2+) state. The reduction of the Fe3+ decreases the methemoglobin level and allows for the reversible binding of oxygen to prevent further tissue hypoxia. Below is a graphic that I made for you visual learners:
Pharmacokinetics of Methylene Blue
Onset of action: 30-60 minutes
Time to peak: 30 minutes
Protein binding: 94%
Metabolism: first-pass metabolism
Half-life: 5-7 hours
Excretion: bile, feces, and urine
Given methylene blue’s rapid onset of action and time to peak, MetHb level reduction is typically seen within 30-60 minutes of methylene blue administration. This rapid resolution of methemoglobinemia is really why this agent remains the primary choice for pharmacological management.
So is it all good? Nope, methylene blue has limitations as well. Let’s review.
Methylene Blue Contraindications
Active use of serotonergic agents
Methylene blue shares a similar chemical structure to monoamine oxidase inhibitors (MAOIs). Therefore, administering methylene blue to patients that are chronically and actively on serotonergic agents can increase the risk of developing serotonin syndrome.
Pregnancy
Methylene blue administration may be harmful to the baby and could lead to intestinal atresia.
Renal failure
Methylene blue should be avoided in patients with severe renal impairment and administered cautiously in patients with mild to moderate renal impairment.
G6PD deficiency
This is an absolute contraindication. Patients with known or a family history of G6PD deficiency should never receive methylene blue as it can worsen methemoglobinemia and lead to death.
Ascorbic Acid (Vitamin C)
Ascorbic acid should primarily be used as a second-line option for patients with severe or symptomatic methemoglobinemia when methylene blue is contraindicated or unavailable. Its mechanism of action isn’t greatly understood, but it has been shown to reduce the methemoglobin formation within 1-3 days of administration.
Given the lack of literature and guideline recommendations, dosing is fairly controversial and not fully known. Usual doses range from 1-10 grams IV every 6 hours until MetHb levels normalize. One of the most common adverse events associated with ascorbic acid use includes the risk of exacerbating kidney stones and kidney failure in patients taking extremely high doses for a prolonged period of time.
Methylene Blue vs. Ascorbic Acid for Methemoglobinemia
The Tl;dr of Drug-Induced (Acquired) Methemoglobinemia
Methemoglobinemia is a rare blood disorder associated with the change in the heme iron configuration from ferrous (Fe2+) to the ferric (Fe3+) state. Oxygen is unable to bind to iron in the ferric (Fe3+) state, which can lead to tissue hypoxia. Common drugs known to induce methemoglobinemia include:
Antimalarials: primaquine, chloroquine
Antineoplastic agents: cyclophosphamide, ifosfamide
Analgesics: acetaminophen, celecoxib
Antibiotics: sulfonamides, nitrofurans
Anesthetics: benzocaine, prilocaine
Others: phenazopyridine, dapsone, nitroglycerin, metoclopramide
Methylene blue remains the mainstay of therapy and should be initiated for patients with a methemoglobin level >30% OR a methemoglobin level between 20-30% in patients who are symptomatic and/or have underlying cardiac or pulmonary disease. Potential contraindications for the use of methylene blue include…
G6PD deficiency
Renal failure
Pregnancy
Concomitant use of serotonergic agents
Ascorbic acid should be utilized as a second-line option when methylene blue is contraindicated or unavailable.
And BAM! There’s methemoglobinemia in a nutshell.