What Every Pharmacist Needs to Know about RSV Prevention
Steph’s Note: Welcome back and Happy New Year!!! To celebrate the start of 2024, we’re here with a massive clinical trial knowledge drop full of information to help protect Baby New Year (and his grandparents) from RSV. If 2020 was the “year of NMOSD” (it’s not all about you, COVID), then 2023 was the “year of RSV”. Here to provide you with all the RSV info is Carley Moses, PharmD. You hopefully remember her from her previous posts on adult vaccines, pediatric vaccines, GLP-1 agonists, and chronic care management, and if you don’t, I highly recommend checking out each and every one of those links. Your new year’s resolution is to read ALL of tl;dr pharmacy’s posts, right???
What a cute Baby New Year! (Image)
And btw, we’re putting pharmacy etiquette aside once again in this post and using some brand names where we feel it’s necessary for y’all. It’s kind of hard to keep track of which specific product we’re talking about when more than one is an RSVpreF vaccine… So bear with us, we do have our reasons for using the brand name.
Take it away, Carley!
Hi again, my phavorite pharmacy phriends!! Today we’re tackling the new RSV prevention recommendations. We’re going to dive into the developing world of preventing RSV for our older populations, as well as our latest guidance for the itsy-bitsy babies and their mothers, so stick with me - we’ve got a LOT to cover here.
(Yes, we did cover some of this info in the recent NICU Series Part 5 post, but this post today focuses 100% on RSV and has the clinical trial meat. So you’re definitely gonna want to read both.)
What is RSV?
I guess to start off we must introduce the villain in this story: Respiratory Syncytial Virus–or RSV, as I’m sure you’ve picked up on by now. RSV is a common virus that affects the respiratory system. When I say common, I mean it leads to more than 2 MILLION outpatient doctor visits every year in the United States. So it’s not exactly a rare condition that lurks in the shadows.
Ewwwwww. The droplets. ALL THE DROPLETS. (Image)
RSV causes the whole gamut of lovely respiratory symptoms. We’re talking runny nose, coughing, sneezing, sore throat, headache, you know, the works. Not all that big of a deal…until it becomes a severe case. Then we may see wheezing, high fevers, and labored breathing. No bueno and definitely a big deal.
Like most other respiratory illnesses, it’s primarily spread through respiratory droplets; however, it also survives on surfaces for several hours. Surprise, surprise…face masks and surface hygiene will be some of the most important preventative tools.
So who is most prone to developing those scary, severe symptoms I just mentioned? You guessed it. The tails of the age bell curve: babies and older adults (60+). We’re going to discuss these things in further detail, but our little ones just don’t have the immune system to be able to tackle our big bad villain (RSV) on their own yet, and our older adults’ immune systems are tired and typically battling other things, causing them to have a decreased immune response to RSV. These two populations are most vulnerable to developing severe illness from RSV and may eventually require hospitalization or even ventilation.
We’ve established that RSV is rather common, but how many of those cases turn severe? According to the CDC, there are 60,000-160,000 hospitalizations and anywhere between 6000-10,000 deaths each year from RSV in people aged 65+. Ouch! That’s no joke. As for the baby end of the bell curve, there’s an estimated 58,000-80,000 hospitalizations and 100-300 deaths every year from RSV in children under the age of 5. Again, NO JOKE.
Why haven’t we been talking about RSV as much as the flu??? Probably because there wasn’t a whole lot we could do about it until now. But now that we can (spoiler alert), let’s talk it up!!
Speaking of RSV and the flu…
Here’s where things have been getting a little delulu. Our RSV villain typically follows a seasonal pattern, like most other respiratory illnesses. RSV is most prevalent in late fall to early spring, mimicking the flu season. Well, like with other things, COVID had a different plan for RSV over the past few years. You see, for 2017-2020, RSV showed itself primarily during October through April. Typical RSV timing, right? Well, in April 2020, we all started wearing masks (one of our toolkit tools), which threw our villain off a bit. The RSV peak for 2020-2021 didn’t BEGIN until around May 2021. That’s right, we fought the villain off for what should have been an entire RSV season. Then things got even more weird. The peak began in May 2021 and lasted until around January 2022.
RSV seasons the last few years, for you visual folks. (Image)
So if you’re following me, we had minimal RSV reported from April 2020 until May 2021, then didn’t get rid of it again until January 2022. Tl;dr: the seasons are all jacked up. When the fall of 2022 came around, the virus was trying to play by its old rules; however, its season this time lasted from late August until late December. It's pretty different from the original season of October to April, right?
Basically, all of this means that it’s getting harder to predict RSV season. We used to be able to know that, when flu season came around, we needed to step up our RSV prevention measures; however, the risk can’t be confined to a seasonal period, at least for right now. This is where our push for new RSV preventative measures in our babies and new RSV vaccines for our older adults has been worked on diligently behind closed doors as COVID-19 took the spotlight.
RSV in Babies
So, what makes our little ones so susceptible to RSV? We’ve technically discussed this before in our article on Pediatric Vaccines, but I’ll give you another quick rundown. When babies are born, they get some antibodies from their mom–IgG. These antibodies are the building blocks of our baby’s immune system and will protect them for about 6 months.
Around this time, the baby’s immune system starts to ramp up production of the other antibodies they need–their own IgG, IgM, and IgA. Their naive T-cells are also learning how to defend them during this time. Basically, from birth until two years old, all of our little ones are still building the army they need to protect themselves, making it PRETTY hard to fight off villains like RSV.
(Image)
You know what doesn’t help?
Where are babies during this time period? They’re at daycare, or with the babysitter, or in pre-school…wherever they are, there are usually other snotty kiddos. How does RSV spread again? Oh yeah, respiratory droplets… Yaaaaaay.
So you’re seeing where this is a problem, right? As we discussed earlier, RSV can cause severe illness in babies, but our babies don’t have the army they need to protect themselves yet. AND they’re at high risk of being exposed to RSV, just by the nature of being a kid! This is where two of our newest RSV tools come in–Beyfortus (nirsevimab-alip) and Abrysvo.
Before we dive into what’s new, it’s always good to reflect on where we came from. Before these new developments, our poster child for RSV prevention in infants was Synagis (palivizumab). Palivizumab is a monoclonal antibody that binds to the fusion protein on the RSV virus itself, making it difficult for the virus to fuse with and enter our baby’s cells and replicate.
While effective, palivizumab was only indicated for:
Premature (</= 35 weeks) infants younger than 6 months at the start of the RSV season
Infants with bronchopulmonary dysplasia (BPD) that required medical treatment within the previous 6 months and are 25 months or younger at the start of RSV season
Infants with hemodynamically significant congenital heart disease (CHD) who are also 24 months or younger at the beginning of RSV season
Pretty strict, right? You would be correct. Palivizumab requires a weight-based dosing approach and monthly injections throughout RSV season, making it a rather laborious prevention method for a relatively small population. Did I mention the cost? It’s hard to nail down an exact cost given the variability in dosing–but the price without insurance comes to a couple thousand per dose per infant.
This is where our first new tool comes in–nirsevimab or Beyfortus. Nirsevimab has the same mechanism as palivizumab–binding to the fusion protein of RSV. However, it has a longer duration of action, decreasing the need for those numerous injections all season. Let’s dive in and meet our new friend now.
Nirsevimab for RSV Prevention in Children
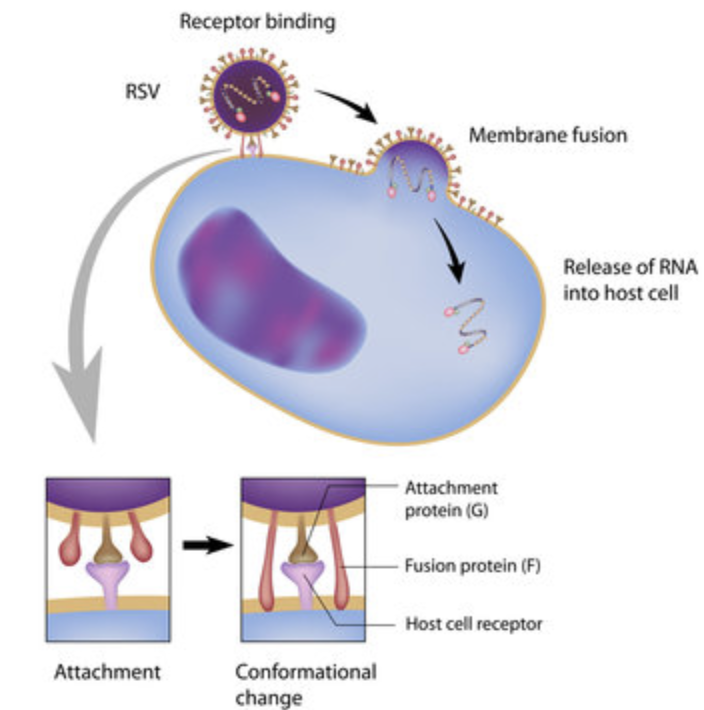
Just to be thorough, let’s take a deeper look at how nirsevimab (and, by association, palivizumab) works to prevent RSV.
(Image)
As we mentioned, on the surface of RSV, there are various proteins that help it latch onto host cells, ultimately entering them to replicate and survive. (Darn hijackers.) Of these proteins, the main ones involved in attachment and replication are the F (fusion) protein and the G (attachment) protein. The F protein exists in its pre-fusion state; it’s floating around space, waiting to be interacted with before making a conformational change to fuse with the host cell. So once the RSV virus finds a host cell, the F protein latches on to a host cell receptor, starting the fusion process. From here, the G proteins latch to the host cell to be nice and sturdy, which tells the F protein, “Alright, dude, we’re good to go.” This is when the F protein undergoes a complete conformational change, ultimately fusing the virus with the host cell’s membrane.
Did you get all that?
Basically, if we can stop the fusion before the F protein starts to change out of its pre-fusion state for attachment, we can prevent infection and replication. That’s where palivizumab, and now, nirsevimab, join the party. They bind to that pre-fusion F protein, never giving it a proper chance to start the fusion process. Pretty cool, right?
For now, a lot of our focus on RSV prevention has been on this F-protein process, and it’s primarily what we will focus on today; however, we will gloss over a few things in the pipeline that look at G proteins, too, just so they don’t get FOMO.
Now that we know how our new tool, nirsevimab, works, let’s get into the nitty-gritty of how it’s changing the RSV prevention landscape.
Let’s put our journal club hat on; we’re diving into the spooky world of clinical trials. Beyfortus (nirsevimab) was approved by showing safety and efficacy across three clinical trials I can’t wait to tell you about.
Trial #1: Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants
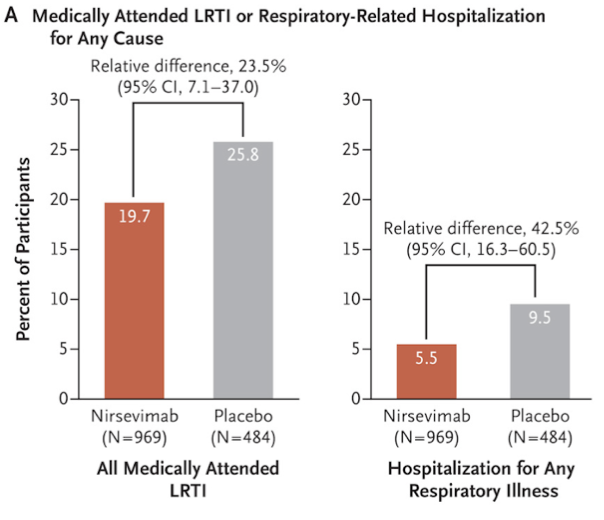
This study was a Phase 2b double-blind, randomized trial completed at 164 different sites in 23 countries. They enrolled 1,453 babies between 29 weeks and 34 weeks 6 days gestational age at a 2:1 ratio, with 969 getting a single injection of nirsevimab 50 mg and 484 getting a placebo. Follow-up was robust, with 98.3% of babies completing 150 days and 91.7% completing a full year of observation.
Regarding outcomes, only 2.6% of the nirsevimab group needed medical attention for RSV-related issues, compared to 9.5% in the placebo group. That's a 70.1% reduction in incidence. Hospitalization? Even better. Just 0.8% of the nirsevimab babies ended up in the hospital for RSV, compared to 4.1% in the placebo group—a 78.4% reduction in risk.
(FYI, those risk reduction numbers are impressively large. Newsworthy, for sure. But don’t forget to account for the difference between absolute risk reduction and relative risk reduction! And if you have forgotten the difference, check out our mini-series on How to Be Awesome at Biostatistics and Literature Evaluation.)
Over the entire 150-day period after getting the single dose, the nirsevimab group was basically thriving. They had a significantly lower risk of needing medical attention for RSV-related issues (hazard ratio of 0.26) and a lower risk of hospitalization for the same (hazard ratio of 0.19).
The gap between the nirsevimab and placebo groups just kept widening over those 150 days, too. It's not just a quick win. It's a sustained protection that lasts the entire RSV season.
Check out how early those curves diverge. The benefit of nirsevimab is apparent soon after administration, and it continues to provide benefit over the entire interval. (Image)
The study didn't just look at RSV, though. It also examined how nirsevimab impacted other respiratory issues. Medically attended lower respiratory tract infections from any cause occurred in 25.8% of the placebo group and 19.7% of the nirsevimab group. That's a 23.5% lower incidence with nirsevimab, making it a multitasker in respiratory protection.
Not really sure how to wrap my head around this mechanistically since nirsevimab is specifically targeted to the RSV F protein…but maybe there’s just something we don’t know yet that could explain this finding! Regardless, the good news is that preventing RSV infection didn’t allow for other lower respiratory tract pathogens to take over and increase their own infections.
Hospitalization due to any respiratory illness was also lower with nirsevimab—5.5% compared to 9.5% in the placebo group. That's a 42.5% lower incidence, so not only does nirsevimab keep RSV at bay, but it also reduces the risk of ending up in the hospital for any respiratory issue. Again, not sure how this works from a mechanism perspective, but I’ll take it!
Adverse events of particular interest were pretty rare—only 0.5% in the nirsevimab group and 0.6% in the placebo group. And what were these events? Mostly just mild stuff like rashes in four participants and petechiae (tiny, flat purple or red spots) in one participant in the nirsevimab group. The placebo group also had three participants with rashes. Notably, there were no anaphylaxis or other scary hypersensitivity reactions. All these events were grade 1 in severity, like the "meh" level of side effects.
Now, let's talk about the elephant in the room: deaths. Five deaths occurred through day 361—two in the nirsevimab group and three in the placebo group. But here's the crucial part: none of these deaths were related to RSV or the treatment. So, while it's a somber note, it doesn't throw shade on nirsevimab's safety profile.
Okay. That was a lot. Here’s the tl;dr from our first trial:
Nirsevimab is not only effective in preventing RSV infection that requires medical attention in premature babies, but it also carries a mild side-effect profile, has a duration that covers the entire RSV season with a single dose, and may (somehow) protect the lungs against other severe respiratory illnesses too!
Trial #2: Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants
In this Phase 3 trial, conducted across 150 sites in 30 countries, researchers enrolled healthy kiddos born at or after 35 weeks of gestation. The study was randomized and double-blind, with 987 infants getting a dose of nirsevimab and 491 receiving a placebo. The dosage varied based on weight: 50 mg for those under 5 kg and 100 mg for the chunkier babies. The study had strong follow-up rates too, with 98.3% of infants tracked for 150 days and 91.7% followed for an entire year.
So, what's the verdict?
A mere 1.2% of the nirsevimab crew had to seek medical help for RSV-related lower respiratory issues, compared to 5.0% in the placebo group. That's a jaw-dropping 74.5% drop in incidence. Regarding hospital stays, nirsevimab also flexed its muscles, as usual. Only 0.6% of the nirsevimab little ones had to be hospitalized for RSV, versus 1.6% in the placebo group. While the 62.1% reduction wasn't statistically significant, it's still something to ponder.
During the 150-day observation window, the nirsevimab group was again ahead of the game. According to a time-to-event analysis, these babies had a markedly reduced risk of requiring medical care for RSV-associated lower respiratory tract infections, boasting a hazard ratio of 0.23.
Again, the protection from nirsevimab against RSV is both early and sustained. (Image)
We also saw, again, that nirsevimab doesn’t just protect our little ones from RSV. In a post-hoc analysis, the risk of hospitalization for any respiratory illness was 59.0% lower in the nirsevimab group. So, it seems that nirsevimab may offer additional protection beyond just RSV, although we’re not really sure how that is just yet…
What about adverse effects? It's still looking good for nirsevimab! Serious adverse events were rare, occurring in 3.6% of the nirsevimab group and 4.3% of the placebo group. The only "special interest" event was a grade 3 rash in one nirsevimab baby, which cleared up on its own. There were no anaphylaxis or other freaky hypersensitivity reactions to report.
Again, I’m giving you a lot of information here. Here’s the tl;dr from our second trial:
In this trial, nirsevimab proved it's not just for preemies. It significantly reduces the risk of severe RSV infections in healthy late preterm and term infants, and it does so with minimal side effects.
Trial #3: Safety of Nirsevimab in High-Risk Infants with Heart or Lung Conditions or Born Prematurely
In this Phase 2-3 trial, the spotlight was on high-risk infants who were split into 2 cohorts—those born prematurely (at less than 35 weeks of gestation) and those with heart or lung conditions, as discussed before. The study was randomized and double-blind, with infants either getting a single dose of nirsevimab (50 mg for those under 5 kg and 100 mg for the bigger babes) or monthly doses of palivizumab, the current standard of care.
The enrollment was robust, with 925 babies in total. Of these, 310 were in the heart and lung disease cohort (CHD–CLD), and 615 were born prematurely. Follow-up was solid, with nearly 96% of infants tracked until day 151 and about 40% followed up to a full year.
First up, let's talk RSV. Only 0.6% of the nirsevimab infants needed medical attention for RSV-related lower respiratory issues, compared to 1.0% in the palivizumab group. Although the full statistical analysis has not yet been published, these results suggest that nirsevimab is at least as effective as palivizumab at preventing severe RSV infections in this high-risk population.
Now, onto safety. Adverse events were pretty much the same across all groups, just like before. There were two "special interest" events in the nirsevimab group and five deaths, but none were linked to the treatment. So, on the safety front, nirsevimab is still looking solid.
This one wasn’t quite as bad, but you know the deal. Here's your tl;dr for our third trial:
Nirsevimab continues to prove its worth, even in high-risk groups. It offers strong protection against severe RSV infections and has a safety profile on par with palivizumab, the previous standard. Whether your kiddo is a preemie or has heart or lung issues, nirsevimab is shaping up to be a reliable protector for those little lungs.
Recommendations Concerning Use of Nirsevimab for RSV Prevention
As of September 2023, the CDC and ACIP recommend that all babies younger than 8 months born during or entering their first RSV season receive one dose of nirsevimab. They go on to reinforce that babies/kiddos aged 8 through 19 months who have an increased risk of severe RSV (chronic lung disease, cystic fibrosis, immunosuppression, etc.) who are entering their second RSV season should also receive a dose of nirsevimab. Dosing follows what we saw in clinical trials: a singular intramuscular injection of 50 mg for babies weighing less than 5 kg and 100 mg for the 5 kg and over little ones.
Additional Considerations Regarding Nirsevimab Use in Infants
First, if you go to Team Nirsevimab, don't even think about adding palivizumab to the mix later in the season. On the other hand, if you started with palivizumab but didn't complete the 5-dose regimen, switch gears and give your infant a single dose of nirsevimab for that RSV season. No more palivizumab after that, capiche?
Now, if your child got palivizumab last season and is still eligible for RSV protection this season (high-risk babies, we see and love you), make the switch to nirsevimab if you can. If it's not available, stick with palivizumab as you did before. And spoiler alert, nirsevimab is in HIGH demand, which has created a shortage of available product. (It’s not just Wegovy making waves in the high demand shortage category this year…)
Another consideration is that timing is everything. Aim to administer nirsevimab in the first week of life for newborns arriving just before or during RSV season. If your baby has a prolonged hospital stay, get that nirsevimab shot in before or right after discharge. For all the other babies who are already home and younger than 8 months or those between 8 and 19 months who are at higher risk, get that shot in right before RSV season kicks off.
And hey, if you missed the boat or the RSV season is particularly unpredictable, nirsevimab can still be given to age-eligible kiddos at any time during the season. But remember, only the high-risk tots should get more than one dose across two RSV seasons. Who's on the high-risk list for a second dose in their second RSV season?
Children with chronic lung disease of prematurity who required medical support (chronic corticosteroid therapy, diuretic therapy, or supplemental oxygen) any time during the 6 months before the start of the second RSV season.
Children who are severely immunocompromised.
Children with cystic fibrosis who have manifestations of severe lung disease (previous hospitalization for pulmonary exacerbation in the first year of life or abnormalities on chest imaging that persist when stable) or have weight-for-length that is <10th percentile.
American Indian and Alaska Native children (note that this is a new group for whom second-season prophylaxis is recommended in contrast to the current palivizumab recommendations).
Geographically speaking, most of the United States should aim for nirsevimab administration from October through March, in line with other vaccines like influenza. But keep an eye on local RSV patterns; you might need to adjust the timing in events like a global pandemic.
Sorry, I'm still recovering from 2020.
Last but not least, don't sweat it regarding giving other childhood vaccines simultaneously. As far as we know, nirsevimab plays well with others. According to the CDC, you can co-administer it with other routine childhood vaccines without compromising effectiveness.
Abrysvo for RSV Prevention
Now, our second new tool works totally differently. Remember when we talked about babies and those antibodies they get from mom when they’re first born that last about 6 months? We can use that science to our advantage to protect our little ones.
You see, if we vaccinate a pregnant mom and mom’s full-grown immune system can make lots of antibodies, those antibodies should then make it to the baby, right? Right. Passive immunity at work! The new RSV vaccine, Abrysvo, does just that when we vaccinate moms during pregnancy to protect babies until they can build their own army.
Now, let's dive into the mechanism of our new RSV tool, shall we?
So, this RSVpreF vaccine, or Abrysvo, is the name of our new tool, and antibody production is its game. This bad boy is a bivalent vaccine, meaning it has two main components that target the two prominent RSV subgroups, RSV A and RSV B.
The show's star is the same F protein we discussed before, specifically in its pre-fusion form, or preF (get it…pre-fusion + F protein…preF) for short. Just in case you’ve forgotten after reading all that clinical trial info, this is the state where RSV’s F protein naturally exists, just floating around waiting to make its grand entrance…or grand fusion.
Here's where it gets cool: the RSVpreF vaccine gives your body a sneak peek of this pre-fusion state F protein so your immune system can recognize it and go, "Hey, I know you! You're not welcome here!" before it even gets a chance to start the fusion process. It does this through developing antibodies to that preF protein, which then bind with the F protein and inhibit the virus from ever being able to attach and fuse with the cell. (This should look familiar–this is how our other antibody tool, nirsevimab, prevented fusion)!
This is like giving your immune system the cheat codes to RSV.
So, how is the baby protected? When a pregnant mom gets Abrysvo, her immune system starts cranking out these RSV-blocking antibodies. And guess what? These antibodies don't just stay with mom. They take a little trip through the placenta to baby town. It's like mom's immune system is handing down a family heirloom of protection to the next generation. So, not only does mom get the cheat codes, but she gets to share them with her newborn. Talk about a win-win!
Long story short, Abrysvo allows mom to build antibodies that block RSV from fusing with her host cells, similar to how nirsevimab works. These antibodies will not only protect the adult mom but will be passed on to the baby, too! Double the human protection–one vaccine. That may or may not be foreshadowing for another population we use Abrysvo in, too…hehe.
Alright, you know the deal. We learn how it works, then we talk about trials. Put that journal club cap back on–time to see how Abrysvo stood up in the trials.
Trial #1: Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants
This Phase 3 trial was a big boi, running from June 2020 to October 2022 and involving a whopping 7,358 women. (Sure, compared to the big cardio or diabetes studies this may not seem like a lot of participants. But for a study involving vaccination of pregnant women? WHOA. Like, whooooaaaaa.)
The study was randomized and double-blind, spanning 18 countries, with 3,682 moms-to-be getting the RSVpreF vaccine and 3,676 receiving a placebo. The follow-up was pretty good, with 85% of the scheduled 180-day follow-up data obtained.
So, how did the vaccine do?
Within 90 days after birth, only 6 babies from the vaccine group needed medical attention for severe RSV-associated lower respiratory issues, compared to 33 in the placebo group. That's a staggering 81.8% drop in the incidence of severe illness. Extend that to 180 days, and the vaccine still held its ground with a 69.4% efficacy rate. So this tells us that the vaccine is REALLY good at preventing those scary symptoms of severe infection we discussed earlier, like respiratory distress.
But wait, there's more.
For general RSV-associated lower respiratory issues, like your usual cough and cold, the vaccine group saw a 57.1% reduction within 90 days of birth and a 51.3% reduction within 180 days. Hospitalization rates? The vaccine flexed again. We're talking a 67.7% reduction within 90 days and a 56.8% reduction within 180 days.
(Image)
Pay attention to that 180-day date–remember when we discussed little baby immune systems? Well, 180 days is around when your babe will start making its own antibodies. This data lets us know that our little one is protected from both severe and general illness all the way until they open their own little antibody factory.
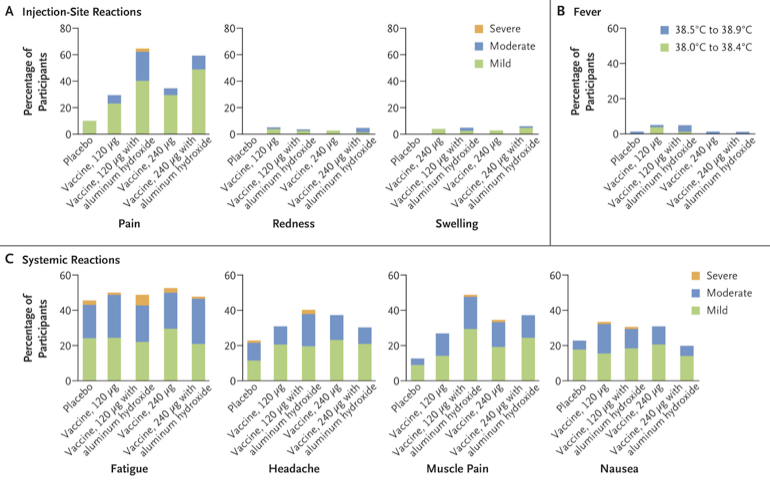
What about adverse effects? Pretty chill, actually. The most common was injection-site pain, reported by 41% in the vaccine group and just 10% in the placebo group. Systemic issues like muscle pain and headaches were slightly more common in the vaccine group, but nothing to lose sleep over.
In general, adverse events were few and far between, thankfully. About 13.8% of the vaccine group and 13.1% of the placebo group reported some sort of adverse event within a month post-injection. For the little ones, it was 37.1% and 34.5%, respectively. What about the serious stuff? It was rare and evenly spread across both groups. One unfortunate death occurred in the vaccine group due to postpartum hemorrhage and hypovolemic shock, but it wasn't linked to the vaccine.
Alright, you've been patient. Here's your tl;dr for this trial:
The RSVpreF Abrysvo vaccine shows some solemn promise. Administered to moms-to-be, it dramatically reduces the risk of severe RSV infections in infants and has a pretty mild side-effect profile. While it doesn't eliminate all respiratory issues, it's a significant step forward in keeping those tiny lungs safe. (Especially since nirsevimab is experiencing a shortage due to demand…)
Interim Analysis: Prefusion F Protein–Based Respiratory Syncytial Virus Immunization in Pregnancy
Heads up fam, this isn't an entirely new show; it's more like a sequel. This interim analysis is a continuation of Abrysvo’s Phase 2 trial. Same participants, new results. Kinda like catching up with old friends, right?
So, this analysis is a multi-country affair featuring moms-to-be between 24 and 36 weeks pregnant, and their newborns got in on the action, too. Blood samples were collected from both parties to see how those antibodies were holding up across multiple points following mom’s vaccine dose, and the kiddos were monitored for up to a year after birth.
Now, let's get to the good stuff. The vaccine showed an efficacy of 84.7% against medically attended RSV-associated lower respiratory tract illness and 91.5% against severe cases. That's not just good; that's "cancel *most* of your worry plans" good. Moms showed 50% neutralizing antibody geometric mean titer ratios ranging from 1:11.0 to 1:15.1 for RSV A and 1:13.7 to 1:17.5 for RSV B. The kiddos weren't far behind, with ratios between 1:9.7 to 1:11.7 for RSV A and 1:13.6 to 1:16.8 for RSV B.
Essentially, these numbers mean that, compared with the placebo group, both the moms who received the vaccine directly AND the babies who received transplacental antibodies from mom had more RSV-targeted antibodies in their blood. Although it’s not totally established what the protective threshold is for this titer, the fact that BOTH moms and babies had several times more antibodies compared with the placebo group is very encouraging!
The most common side effect was mild-to-moderate pain at the injection site, especially for those who got the vaccine formulated with aluminum hydroxide. Fever? Rare. Only about 5.1% had a temp of 38.0°C or higher. Serious adverse events were a rare sight, too, and none were linked to the vaccine. Adverse events were primarily typical neonatal stuff like jaundice or minor gastrointestinal issues for the little ones.
Not much orange on these charts, meaning there were few severe adverse effects. (Image)
WE’RE ALREADY BACK TO MY FAVE PART! Here’s your tl;dr:
Abrysvo vaccine is doing its thing, boosting antibody levels in both moms and their little ones and preventing both RSV infection that requires medical attention and severe RSV infection. Adverse effects are minimal, and the safety profile is solid. This vaccine is shaping up to be a key player in the fight to keep those baby lungs healthy.
That being said, we would be remiss if we didn’t at least mention this…
According to the package insert, Abrysvo shows a higher incidence of low birth weight when compared to a placebo. While this seems scary, the trials had insufficient data to say that Abrysvo was the cause of these rates, so just keep this info handy when advising expectant moms.
Recommendations for RSV Prevention Using Abrysvo in Pregnancy
As of October 2023, the CDC and ACIP recommend the RSVpreF vaccine Abrysvo for pregnant persons at 32–36 weeks’ gestation using seasonal administration (meaning September–January in most of the United States) to prevent RSV-associated lower respiratory tract infections in infants aged <6 months. Abrysvo is administered intramuscularly as a single 0.5 mL dose; beware, this vaccine does require reconstitution. Refer to the package insert for a handy-dandy step-by-step picture guide!
Considerations for Using Nirsevimab Versus the RSVpreF Vaccine
But wait–we just learned all about nirsevimab, which has a very similar role to Abrysvo. So what should we do? Well, as usual, there are pros and cons to the use of both.
First and foremost, always follow whatever guidelines and recommendations are in place. We play nice here. With that being said, if your patient is uninsured or underinsured, Abrysvo may be a more affordable option for them. Another advantage of Abrysvo is that it provides protection against RSV from birth rather than after the time of injection. If a baby is born during RSV season, this immediate protection may be necessary; however, if the baby comes significantly before or after RSV season, antibody levels may have already waned before your baby really gets to use that protection.
On the flip side, nirsevimab can be timed before a little one’s highest chance of exposure. This allows us to protect them for the duration of the RSV season. Secondly, relying on the maternal vaccine for protection also requires sufficient transfer of antibodies from mama to baby through the placenta. In scenarios such as premature birth, poor perfusion for mom, or even a birth soon after vaccination, the tater tot may not have gotten sufficient antibodies yet to truly keep them safe.
Milk coma! So darn cute <3 (Image)
Basically, if nirsevimab is available, it is the new gold standard for RSV prevention. Its convenience of a single dose, long duration, ability to target a specific season, robust response, and mild side effect profile make for the perfect prevention cocktail to put baby in a milk slumber. That’s not to knock on Abrysvo; it could be an excellent option for our uninsured, underinsured, babies who need protection immediately after birth, or patients who may have difficulty returning for the nirsevimab injection. Regardless, the goal here is to keep them little babies healthy!!
You thought we were done with Abrysvo, didn’t you…
I love a good plot twist!!! Before we continue our Abrysvo parade, let’s shift gears. I foreshadowed this a little bit earlier…an Easter egg, some may call it… Let’s talk adults.
Remember when I said that Abrysvo also mounted an immune response in adults? We saw the titer ratios, which told us mom, an adult, was also protected? Well, we mentioned adults somewhere else earlier, too. I think it was when we were talking about high-risk groups and RSV…
THAT’S IT!!!
You see, if Abrysvo was able to protect our adult mamas from RSV, it theoretically should offer some protection for the high-risk, 60+ adults, too…right?! That’s the hope!
We’ve talked a lot about immune responses today, so I won’t drag you through the entire adult immune response–BUT–it is essential to understand that multiple chronic conditions can really take a toll on one’s ability to fight RSV. The patients we will focus on from this point forward are those with lung disease, cardiovascular disease, diabetes, you get the picture. The ones who may need a little extra pep in their step to safely bring them through RSV season–CUE THE JOURNAL CLUB CAPS!
Trial #3: Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults
We're talking about the RENOIR study, another Phase 3, double-blind, randomized trial here. This one was completed in seven countries with a whopping 35,971 participants aged 60 or above. The randomization was at a 2:1 ratio; two-thirds got the RSVpreF vaccine, Abrysvo, while the other third was given a placebo.
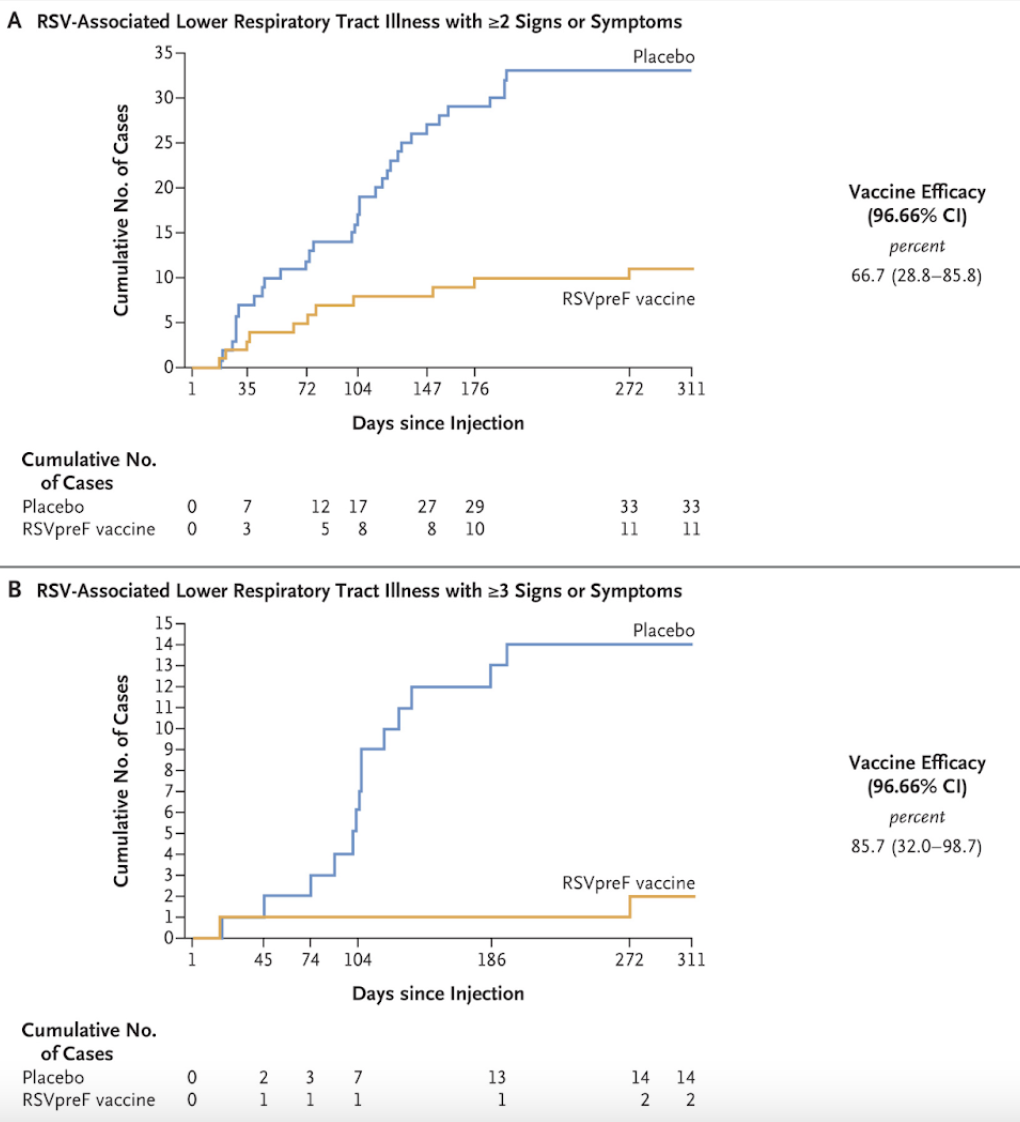
These Kaplan-Meier curves really show the difference - the protection from vaccination is fast and prolonged based on the divergence of the curves! However, it’s still unclear how durable the protection is from year to year or how robust it is in those with compromised immune systems. (Image)
Let's cut to the chase; we’ve done this enough times now. Efficacy. After day 15, the vaccinated group showed a 66.7% reduction in RSV-associated lower respiratory tract infections with 2 or greater symptoms. Symptoms hitting you like a truck? Abrysvo's effectiveness soared to 85.7% for preventing infections with three or more symptoms. Finally, when considering RSV-associated acute respiratory illness as a whole, Abrysvo boasted a 62.1% efficacy, with most of those acute illnesses being caused by RSV B.
In a subgroup of 7,169 participants, local reactions were a bit more common in the vaccine crowd at 12% compared to 7% in the placebo group. Systemic events? Virtually a tie, at 27% for the vaccine group and 26% for placebo. Severe events were rare, showing up in less than 0.7% in both groups.
Adverse events within a month of the jab were pretty balanced, too: 9.0% for the vaccine group and 8.5% for the placebo. Most of these were mild, like infections and respiratory issues. Serious adverse events were a flat 2.3% in both groups, with three cases considered trial-related: one allergic reaction and 2 cases of symptoms consistent with Guillain–Barré syndrome. All recovered, and no one dropped out due to adverse events.
So, what's the tl;dr????
Abrysvo continues to prove its efficacy in not only our pregnant mamas but also in older adults, too! Safety profile overall is promising, although the higher-than-background rate of Guillain-Barre syndrome means the CDC is conducting post-marketing surveillance for this (and any other neuroinflammatory conditions).
Arexvy for RSV Prevention
Before we dive into our recommendations for our older adults, we will talk about one last new tool you can use. This one, once again, is for our adults 60+ with chronic conditions that may make RSV season a little difficult for them to stick out. Thankfully, the mechanism of immune induction for Arexvy is the same as that of Abrysvo! You guessed it–an excellent, pretty potion of pre-fusion state F proteins just waiting to have antibodies made to cater to them.
There is one difference from Abrysvo though. Arexvy is adjuvanted, meaning it contains a proprietary ingredient aimed at producing a stronger immune response. This can potentially have some impacts on coadministration with other vaccines.
Trial #1: Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults
Alright, let's dive into this Phase 3 monster of a trial that spanned 17 countries and enrolled a jaw-dropping 26,664 participants. First off, the randomization was tight. Participants were split evenly between receiving a single intramuscular dose of Arexvy or a placebo. The average age? About 69.5 years. And get this: around 39% of these folks had some underlying conditions that made them more susceptible to severe RSV. Talk about inclusivity!
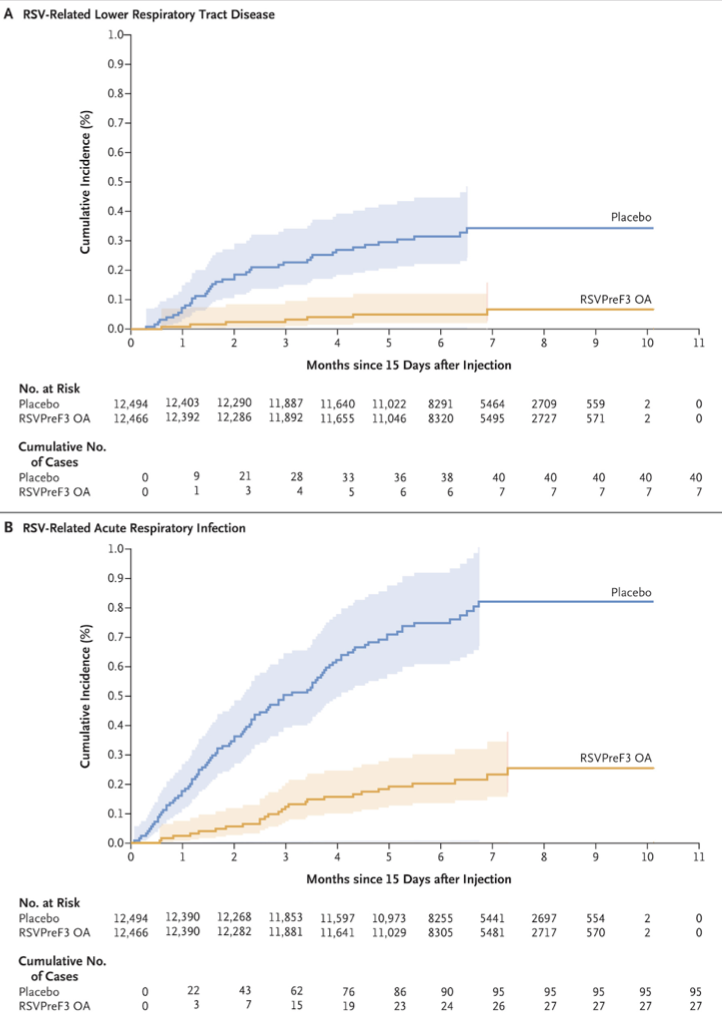
Now, let's talk results. Hold onto your hats because this vaccine is showing off. Within a median follow-up of 6.7 months, only 7 out of 12,466 in the vaccine group reported RSV-related lower respiratory issues, compared to 40 in the placebo group. That's an 82.6% efficacy rate, people! And when it comes to severe cases? The vaccine flexed hard with a 94.1% efficacy rate.
More impressive vaccine Kaplan-Meiers. #govaccines! (Image)
But wait, there's more.
Arexvy also reduced the incidence of general RSV-related acute respiratory infections by 71.7%. And it didn't matter if it was RSV A or B subtype. The vaccine was effective against both.
Side effects, again, were mainly a walk in the park. The most common was injection-site pain, reported by 60.9% in the vaccine group and just 9.3% in the placebo group. Fatigue was also more common in the vaccine group, but nothing major. As for serious stuff, it was pretty balanced. About 4.2% in the vaccine group and 4.0% in the placebo group reported a serious adverse event. Deaths were rare and evenly spread; none were directly linked to the vaccine.
So, your tl;dr for this trial:
Arexvy is a game-changer for older adults. It's highly effective in preventing both mild and severe RSV-related respiratory issues with a generally chill side-effect profile. This could be a big win for our older peeps, especially those with underlying conditions.
Although… unfortunately, Arexvy hasn’t escaped the question of Guillain-Barre syndrome either. Like Abrysvo, it will undergo post-marketing surveillance for this as there were 3 cases of possible neuroinflammatory events within 42 days of administration in other studies of this vaccine. One case was Guillain-Barre, and the other 2 were deemed acute disseminated encephalomyelitis (ADEM). So although these are small numbers, they do still seem to be higher than the normal background rates, which is why additional monitoring is necessary.
Recommendations for Use of Abrysvo or Arexvy in Older Adults for RSV Prevention
As of September 2023, the CDC and ACIP recommend using shared decision-making to determine an individual’s risk and need for the RSV vaccine. They go on to recommend a single dose of either Abrysvo or Arexvy to any individual 60 or older who is considered at high risk of severe illness from RSV before the start of RSV season. Both vaccines consist of a single 0.5 mL intramuscular injection. Remember, both Abrysvo and Arexvy require reconstitution before administration; use those pretty package inserts to help you!
Additional Considerations for RSV Vaccination
First things first, timing. We'd usually say to vaccinate before RSV season kicks in, but let's be honest—COVID has thrown all our calendars out the window, as we discussed. So, for the 2023-24 season, as soon as that RSV vaccine stock hits your fridge, start jabbing those eligible arms. And if someone's late to the game? Keep that vaccine on tap. As the RSV season normalizes again, make it a goal to vaccinate for RSV before the start of the RSV season, similar to the flu vaccine.
Now, the million-dollar question: Can you co-administer the RSV vaccines with other adult vaccines? Short answer: Yes, but with a caveat. The data on co-administration is still in its infancy.
We know that when Arexvy was paired with an adjuvanted flu vaccine, the immune response took a slight hit for one flu strain. (For the record, this was also when the 2 cases of ADEM reared up with Arexvy…) But was it clinically significant? The jury's still out. If you're thinking of double-dosing your patients with RSV and another vaccine, expect a bit more reactogenicity. But let's be clear: this is mainly based on the RSV-flu vaccine combo. For other vaccines like COVID, pneumonia, or even the shingles vaccine, we're flying blind until we get further data.
Considerations of Cost and Insurance Coverage for the RSV Vaccines
Since both of these vaccines are so new, insurance coverage will vary. Thus far, Medicare Part D intends to cover either of the vaccines for their subscribers. Advise your patients to check with their health insurance for correct information about coverage. Without insurance, patients can expect to pay a whopping $180-$330 for a single dose of the RSV vaccine (still less expensive than a hospital admission, though).
On The Horizon for RSV Prevention
Before I sign off and end my RSV-driven tangent, I wanted to leave you with tidbits regarding the future of RSV prevention. Thus far, the available biologics and vaccines have focused on that F protein we’ve talked so much about today.
In the pipeline, there are all sorts of new friends coming, including live-attenuated vaccines, G protein-based vaccines, mRNA vaccines similar to our breakthrough COVID-19 vaccines, adenovirus vector vaccines, and even more unique and improved monoclonal antibodies. With this being said, let this serve as a starting point for your RSV prevention adventure; be sure to stay up-to-date with rapidly advancing technologies so you’re ready to pivot for the safety of those at risk!
Alright, you guys, that was a lot. I don’t know about you, but I am TIRED, so let’s get to this final tl;dr…
The tl;dr on RSV Prevention
It’s been a revolutionary time in our strides to protect babies and older adults during RSV season. New developments include nirsevimab for our newborn babies, Abrysvo for our close-to-delivery pregnant mamas and older adults, and finally, Arexvy for only our older adults. Use the knowledge you’ve learned today, combined with your CDC and ACIP recommendations, and get out there and protect patients this RSV season!