There's a Fungus Among Us: A Beginner's Guide to Antifungals
Editor's Note: Molly Curran, PharmD, BCPS, BCCCP, is a Clinical Assistant Professor in the Department of Health Outcomes and Pharmacy Practice at the University of Texas-College of Pharmacy and a Clinical Pharmacy Specialist in Internal Medicine at the Dell Seton Medical Center.
Did you just feel yourself correcting your posture and sucking in your gut as you read that? Because I definitely did when I was typing it.
Dr. Curran has a PGY1 and a PGY2 (in critical care) under her belt. She splits her time between teaching at one of the top pharmacy schools in the country and practicing at a brand new, state of the art, Level I Trauma Center in Austin, TX.
And in spite of all of that, she found the time to write this (seriously amazing) primer on antifungals for your learning enjoyment. #Goals. Anyway, let's give her a warm and fuzzy tl;dr welcome!
If you’re like me, during pharmacy school you probably retained two things about antifungals:
Azoles cause crazy drug interactions mediated via CYP450 3A4
Amphotericin kills the kidneys
This likely got you through a few questions in class, but it's no help when you start your acute care APPE rotation and your patient suddenly is infected with Cryptococcus or candida or aspergillus or an endemic fungus of your choice or most terrifying...MUCOR!
Part I: Clinically Relevant Fungal Infections
Before we discuss the major drugs, it’s important to briefly review the most common fungi we encounter clinically. It’s hard to pick the right antifungal agent unless we know what we’re treating!
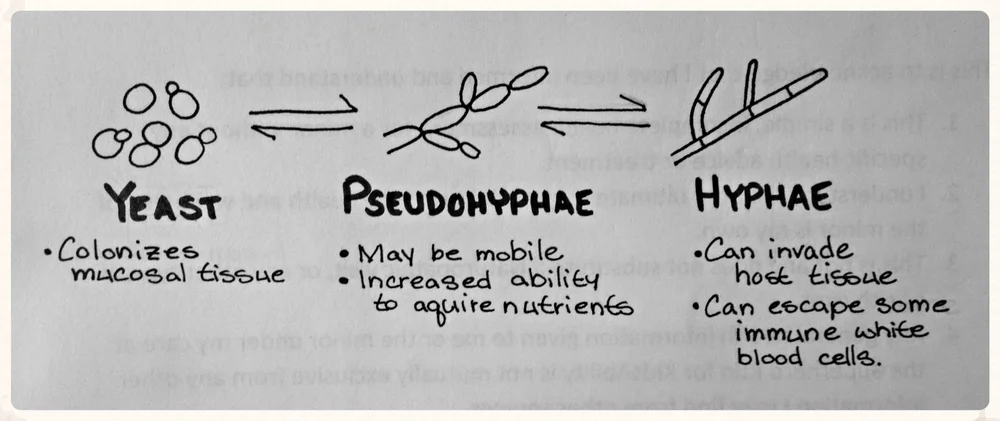
Candida
Candida is probably the most common fungal pathogen. On gram stains, the clinical microbiologist will probably report finding yeast/pseudohyphae under the microscope.
Unfortunately, this isn’t enough to make a good clinical decision about which agent to use for treatment.
After you are suspicious for a Candida infection, it is important to determine which type of species is growing in the cultures. There are 3 different species that are encountered clinically:
Candida albicans
Candida glabrata
Candida krusei
It is important to differentiate which species you are treating because they have very different patterns of fluconazole resistance. Luckily for us, most Candida infections will be due to Candida albicans, which is very treatable with fluconazole.
The two species of Candida to be on the look out for are Candida glabrata and Candida krusei. While less common pathogens, glabrata isolates have been found to have ~10% resistance to fluconazole and krusei isolates are innately resistant to fluconazole therapy.
Candida glabrata: ~10% resistance to fluconazole (check susceptibilities before treating)
Candida krusei: Do not use fluconazole
While any patient may be susceptible to a Candida infection. Candida tends to cause 2 major types of infections:
Oropharyngeal
Occurs in the immunosuppressed patients (think of: oncology, HIV, transplant patients)
Disseminated candidiasis/candidemia
Often associated with certain risk factors: broad spectrum antibiotics, parenteral nutrition, central venous catheter, abdominal injury/surgery, critically ill (in the ICU)
Now let’s briefly run down the other major fungi that you may encounter during your inpatient pharmacy practice.
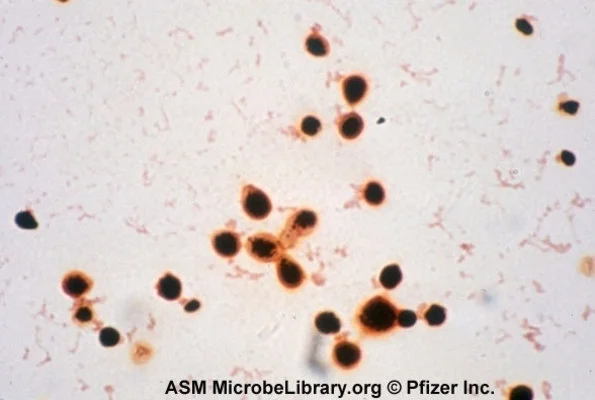
Cryptococcus
Cryptococcus is a fungus that may cause meningitis or fungemia in immunosuppressed patients (most commonly HIV or cirrhotic patients).
It is also classified as yeast on gram stain, but can be identified using India ink preparation, which leaves a halo around the yeast cells (see some cool examples of the halo here).
This is important as it often changes the treatment recommendations (which we'll get to soon).
Endemic Fungi
I know you remember this picture from your ID module... (Image)
Endemic fungi cause disseminated infections primarily in immunosuppressed patients (any themes emerging yet?). Each of these fungi is associated with a different geographic area of the US.
As you can see on the map above, these endemics include:
Coccidioides (aka Cocci) à Found in the desert southwest
Histoplasma (aka Histo) à Found in the Mississippi River Valley
Blastomyces (aka Blasto) à Found in the Mississippi and Ohio River Valley
Molds
Finally, there are two molds to discuss. Both are important to identify and (if acting as pathogen), treat quickly.
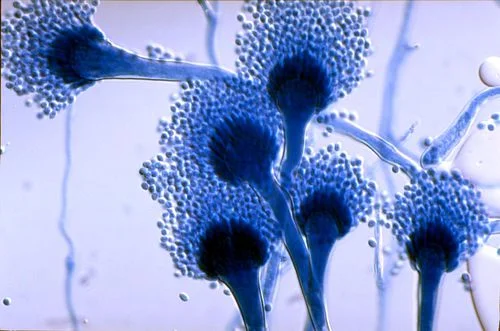
Aspergillus. All of those filament-looking things are hyphae (Image)
First up is aspergillus, which looks like a mold or true hyphae on gram stain.
It typically causes pulmonary infections in immunosuppressed patients (AGAIN!?!).
It's got an especially high affinity for the following groups:
Neutropenic patients (ANC <500)
Lung transplant recipients
Cystic fibrosis patients
Pixar's depiction of Mucorates
Finally, we saved the worst for last: Mucorates (Editor's Note: That just SOUNDS like a villain in a Pixar movie, doesn't it?).
Mucorates (often abbreviated as Mucor) may cause an aggressive infection that necessitates surgical debridement. While rare (thankfully), it typically affects patients who are:
Diabetic
Neutropenic
Sustained trauma
Taking chronic steroids
Now that we’ve reviewed the major offenders when it comes to fungal pathogens, let’s review the arsenal of drugs we have to treat and attack them.
After all, we are pharmacists!
Part II: Antifungal Therapy
Before jumping into treatment, it is important to consider that fungi have cell walls that are constructed as highlighted in the graphic below:
Image
This is relevant to how we treat fungal infections because we can design drugs that target different parts of the fungal cell wall. As you can see in the picture above, every class of antifungal drugs works via a different mechanism.
And because human cells don't have a cell wall, this makes our antifungal treatment relatively well-targeted. For our review today, we will focus on the 3 major types of antifungal medications:
Azoles
Polyenes
Echinocandins
Azoles
Azoles treat fungal infections by blocking the demethylation of lanosterol by binding CYP450. This prevents the formation of ergosterol (again, see the picture above for a visual).
Basically, this means azoles prevent the fungus from creating enough ergosterol to maintain a cell membrane. This will eventually kill the fungal cell, but it takes a little while (compared to some of the antifungals we'll talk about below).
Therefore, you could say azoles are fungistatic and exhibit time-dependent killing of fungi. That's an important concept, so let's repeat it, shall we?
Azoles: Fungistatic, time-depending killing of fungi
There are 5 different azoles to know about (relax, this is the most of any class of antifungals), so let’s get started!
I'll briefly discuss the clinical pearls of each agent and review the major take home points at the end.
Fluconazole
Fluconazole is the drug of choice for Candida albicans, Cryptococcus, and Coccidioides.
It is available as an IV formulation or an oral formulation (both tablets and suspension). It's got great bioavailability (F>95%) and distributes everywhere in the body (think CSF, saliva, tissue, urine).
Overall, fluconazole is well tolerated and requires very little monitoring. Drug interactions are medicated via CYP metabolism (especially 3A4 and 2C9).
Though well tolerated, you should also monitor for a few adverse drug reactions such as elevated LFTs, reversible alopecia and QT prolongation. There's also a renal adjustment to consider for your patients with so-so function kidneys.
Itraconazole
Itraconazole is an oral agent that can be used to treat Histoplasmosis, Blastomyces or as prophylaxis against aspergillus.
Here’s where it starts to get a bit complicated!
Oral itraconazole is available as both a capsule and a solution, but they are NOT interchangeable. The capsule is has ~30% bioavailability and to achieve optimal absorption, it should be taken presence of acid (cola) and food (full meal).
In comparison, the solution has better bioavailability (F~50%), but it must be taken on an empty stomach (>1 hr before or >2 h after meals).
Itraconazole Capsules - Take with acid (such as cola) and a full meal for optimal absorption
Itraconazole Solution - Take on an empty stomach for optimal absorption
Because it is so difficult to absorb itraconazole and it is used to treat important infections, it requires drug level monitoring to ensure that the levels are sufficient to treat the fungi.
After ~ one week of treatment, a serum trough concentration should be obtained with a goal level >0.5ug/mL.
With regards to drug interactions, itraconazole causes more significant CYP3A4 interactions and more serious hepatotoxicity (compared to fluconazole).
See? Look how black that box is... (Image)
It also has two black box warnings.
One, it may cause negative inotropic properties which may lead to chronic heart failure.
And two, because of it's strong 3A4 inhibition, coadministration of itraconazole can cause increased plasma concentrations drugs that prolong the QT interval.
This can lead to ventricular tachyarrhythmias (including Torsades des pointes).
So, long story short, itraconazole needs to be avoided in patients with a history of heart failure or ventricular dysfunction.
Posaconazole
Posaconazole is available IV and PO (tablets and suspension) and is most commonly used as prophylaxis for aspergillus (think patients with cancers such as AML) or maintenance or salvage therapy for mucormycosis.
There are two oral formulations and, like itraconazole, they are not interchangeable.
The oral suspension is hard to absorb because it is highly lipophilic and not water-soluble. It is dosed 2-4 times per day and should be given with a high fat meal or acidic carbonated beverage.
The presence of stomach acid is required for absorption. Acid suppressing agents (such as PPIs and H2RAs, etc...) should not be given to patients on a posaconazole suspension.
To ensure the patient is getting adequate absorption from the oral suspension, therapeutic drug monitoring is essential! The target trough concentration for posaconazole suspension is >0.7 ug/mL for prophylaxis and >1 ug/mL for treatment.
> 0.7 ug/ml for prophylaxis
> 1 ug/mL for treatment
Luckily, a tablet formulation with better absorption was recently approved and it has much better bioavailability. As a result it can be dosed once a day and no therapeutic drug monitoring is necessary.
Like any other azole, posaconazole interacts with drugs metabolized by CYP3A4 or P-gp and can increase the QT interval (so try to avoid it with other drugs that significantly prolong QT).
Based on these drug interactions, you can probably guess what the significant adverse drug reactions are observed with posaconazole are! Did you guess them?
Hepatotoxicity and QT prolongation!
Voriconazole
Voriconazole is the drug of choice for the treatment of invasive aspergillus and may be used as a salvage therapy regimen for other severe mold infections like fusarium.
It is available in IV and PO formulations, but unlike the previous two azoles, it is bioequivalent and can be interchanged. Although the PO formulation efficacy may be affected by the presence of food, so both the suspension and the tablet should be taken on an empty stomach.
Vori (that's the cool kid nickname for it) also requires therapeutic drug monitoring. Specifically, we'll check a trough level after ~7 days of therapy to help us avoid toxicities.
The goal trough concentration for voriconazole therapy is 2-6 ug/mL.
Interactions are similar for voriconazole to other azole agents since they are also metabolized via the CYP 450 system.
In addition to the CYP3A4 effects, voriconazole also is affected by extensive CYP2C19 metabolism. Given the known 2C19 genetic polymorphisms in the population (remember that whole thing about Plavix and the CYP2C19 *2 and *3 alleles?), this also effects therapeutic drug levels and is an important reason to monitor therapy closely.
Additionally, caution should be exercised when co-administering voriconazole with other QT prolonging agents.
But wait, there's more! Voriconazol has more significant adverse drug reactions than any other azole. It causes transient visual disturbances (think "vori and vision") that can be predicted by the “rule of 30’s”:
~30% of patients experience photopsia (appearance of bright lights, color changes, wavy lines, blurred vision)
Lasts ~30 minutes
Abates after ~30 days of treatment
Additionally, vori causes cutaneous phototoxicity (that cannot be prevented with sunscreen), hallucinations, confusion, and rash.
Prolonged use of voriconazole can also lead to several other significant side effects including cutaneous malignancies (squamous cell carcinoma and melanoma), alopecia, skeletal disease of long bones (periostitis and exostoses).
The IV formulation is made with cyclodextrins that can accumulate in patients with underlying renal dysfunction (CrCl < 50mL/min) and lead to nephrotoxicity. Based on these side effects, we do not mess around with voriconazole and reserve it for dangerous infections.
Isavuconazole
Isavuconazole is the newest azole around and is available in an IV and PO formulation. Like voriconazole, the oral and IV formulations are bioequivalent.
It is used to treat invasive aspergillus and mucormycosis infections. Isavuconazole doesn't have as many CYP interactions as vori, but it may interact with strong 3A4 inhibitors and inducers.
In contrast with other azoles, isavuconazole actually SHORTENS the QT interval. This sort of fact makes it an outlier and is exactly the kind of thing that would show up on a test. Just sayin'.
Given its recent approval, we're still learning about the extent of adverse reactions caused by isavuconazole. But there are several big ones to be aware of.
Most importantly, isavuconazole may cause serious hypersensitivity and severe skin reactions. Additionally, it had been shown to cause hypokalemia, peripheral edema and dyspnea.
Annnndddd breathe...
So, those are the azoles!
Key things to remember:
They all are metabolized via cytochrome 450 system and have significant drug interactions with other CYP450 drugs.
Each agent has a distinctive place in therapy and a different spectrum of antifungal activity.
When using itraconazole or posaconazole, pay close attention to the bioequivalency of solutions and tablets because some dosage forms are not interchangeable.
Due to variations in absorption, voriconazole, itraconazole and posaconazole suspension require therapeutic drug monitoring to ensure they are actually working.
For more specifics, I suggest reviewing this excellent article that details the interactions and each agent with much greater detail.
By now, you are probably feeling like:
Get up, stretch and come back while we break down the ins and outs of two more important classes of anti-fungal therapy.
Polyenes
Polyenes treat fungal infections by directly binding the ergosterol in the fungal cell membrane to disrupt permeability and rapidly cause cell death.
As a result, they exhibit concentration dependent fungicidal killing.
Polyenes: Fungicidal, concentration dependent killing of fungi
Within the class, there two agents that we encounter clinically:
Nystatin
Amphotericin B
There isn't a whole lot to say about nystatin. It is most commonly used as an oral rinse for oral thrush (Candida) infections. You can either swish and spit or swish and swallow (depending on how far back in the throat the infection goes) for 3 - 4 times daily. It's is also used for some topical infections.
No, for this article, we will focus on amphotericin B or ampho “terrible.”
This agent is typically used to treat cryptococcal and severe endemic infections and may be salvage therapy for the treatment of fusarium and aspergillus.
The first thing to know about amphotericin B is that it is ONLY available IV. Oh goodie! Finally something simple, you say? Not quite...
There are 4 different IV formulations to be aware of and the dosing is NOT equivalent. The 4 formulations available are:
Deoxycholate (conventional amphotericin B) = CAB
Lipid complex = ABLC
Liposomal = L-AMB
Cholesteryl sulfate complex = ABCD
Luckily, 3 of these are lipid formulations (ABLC, L-AMB, ABCD) and can be considered equivalent for dosing purposes.
So, basically if a provider is ordering amphotericin, you need to know whether they want to order conventional (CAB) or one of the lipid formulations to interpret the appropriateness of dosing. Most institutions will only have one of the lipid formulations on formulary, so this will help streamline your decision.
While, amphotericin does not require drug level monitoring, it does cause profound nephrotoxicity. So we do monitor therapy, just in a slightly different way. We watch for signs of nephrotoxicity by checking BUN and SCr throughout therapy to assess for azotemia and tubular acidosis.
In addition, both formulations of amphotericin B (CAB more so than liposomal AB) impair the body’s ability to concentrate the urine. This results in some pretty profound electrolyte wasting (especially K and Mg!), so electrolytes should be monitored and aggressively replaced during therapy.
The nephrotoxicity also means the use of concomitant nephrotoxic medications (think vancomycin, aminoglycosides, NSAIDs, IV contrast, etc...) should be minimized as much as possible.
Finally, CAB and L-AMB may be associated with serious infusion reactions.
The infusion reaction associated with CAB typically causes fever, chills, headache, nausea/vomiting, hypotension, and tachypnea within 1-3 hours after starting the infusion.
To minimize these effects, the patient may be pretreated with acetaminophen and/or diphenhydramine (so it's sort of like a "normal" infusion reaction).
Poor Linus has been stricken with Cryptococcal meningitis and must be started on L-AMB (Image)
The infusion reaction associated with L-AMB causes a triad of symptoms described by patients as a feeling of “impending doom” within 5 minutes of starting the infusion.
These symptoms include:
Crushing chest pain, dyspnea, hypoxia
Abdominal, back and leg pain
Flushing/urticarial
To minimize this reaction, the patient may be pre-medicated with acetaminophen and/or diphenhydramine 30-60 minutes prior to the infusion (just like CAB).
These patients should also receive aggressive fluid resuscitation (500-1000mL of NS).
Other significant side effects to be aware of with CAB include:
Weight loss
Malaise
Anemia
Thrombocytopenia
Cardiac toxicity
Myopathies
CAB also has a long half-life, so these effects may persist for days to weeks after therapy is stopped.
Let’s stop and review the major points of AB therapy!
AB is fungicidal and the drug of choice for treating cryptococcal infections.
Know which formulation you are using because it has important implications for dosing and side effects.
AB may cause nephrotoxicity, so proactively minimize the risk of it by avoiding concomitant nephrotoxic medications, aggressively hydrating and carefully monitoring the BUN/SCr to identify any signs of impending renal dysfunction.
AB impairs the body’s ability to concentrate the urine leading to electrolyte wasting, so electrolyte monitoring and replacement focusing on K and Mg.
Echinocandins
The echinocandins work by inhibiting 1,3-beta-D glucan synthase (which is involved in the synthesis of the fungal cell wall). Inhibiting this enzyme reduces of integrity of the cell wall leading to rupture and death.
Like polyenes, these agents are fungicidal and exhibit concentration dependent killing.
Echinocandins: Fungicidal, concentration dependent killing of fungi
They are primarily used to treat candidemia, but also may be used as alternative therapy in the treatment of aspergillus.
Like the polyenes, echinocandins are only available IV. There are three agents:
Caspofungin
Micafungin
Andidulafungin
All 3 agents are similar in spectrum, efficacy and safety. They are dosed once daily and do not require therapeutic drug monitoring.
It may be helpful to periodically check LFTs with caspofungin and micafungin since they are hepatically metabolized and may cause hepatotoxicity.
Otherwise, these agents have very few toxic adverse drug reactions in humans since they work on the integrity of cell walls, which are not present in human cells.
Basically, echinocandins are a great empiric option for critically ill patients with candidemia based on the lack of side effects and the fact that all the species of candida are predictably susceptible to them.*
*There are some reports of growing echinocandin resistance...for more details check this out.
Antifungal Therapy: Final Points
Antifungals are a varied class of drugs and you need to pay close attention to make sure your patient is receiving optimal coverage!
If you’re thirsty for more information about antifungal therapy or treating fungal infections, may I suggest checking out (plus the references I already listed):