The Pharmacist's Quick(ish) Primer on Status Epilepticus
Steph’s Note: After a couple of weeks’ break from clinical posts, we return today with a clinical goodie - status epilepticus. Sure, it sounds like a spell from Harry Potter, but it’s real. And it’s scary. And it can be real scary. Which is why we pharmacists should know what it is and how to manage it, especially if you have any inklings about working in the inpatient realm.
Here to teach us about this important neurological topic is David Mastro. David is a PGY-1 Pharmacy Resident at Guthrie Robert Packer Hospital in Sayre, Pennsylvania. He’s interested in critical care and emergency medicine, which is of course why this is such a fitting topic for him. Take it away, David!
As High School Musical sang to us over 15 years ago (WOW, I am getting old), “Stick to the status quoooo!”, well… I want you to “Stick to status epilepticussss!”, because clinical pharmacists can play an important role in its management.
Was that a good segue into our topic?
I thought I was being clever with the High School Musical quote, but that didn’t really go anywhere. Anyways, I hope you can learn something from this article and apply it to clinical practice.
What is Status Epilepticus?
Tried to find an Anakin one to make this because yknow he’s beautiful and all, but this fit :) Have a good and seizure free Star Wars Day…! (Image)
Status epilepticus is a neurological medical emergency consisting of dangerous seizure activity that results from either a failure of seizure-inhibitory mechanisms or initiation of seizure-inducing mechanisms. Previously, the Neurocritical Care Society defined SE as seizure activity lasting at least 30 minutes. But their newest definition is the following:
Seizure lasting at least 5 minutes, OR
Recurrent seizure activity (at least 2 discrete seizures) without recovery between seizures.
Status epilepticus can result in long-term neurological sequelae, including neuronal injury, death, and alteration of neuronal networks. This damage may be irreversible and can occur within just 30 minutes of ongoing seizure activity.
Who Experiences Status Epilepticus and Why?
The incidence of status epilepticus has a bimodal age distribution, meaning it is most common in children (less than 4 years old) and adults (greater than 60 years old). Short-term mortality (mortality within 30 days) is highest among the elderly. Check out these additional details about the differences between SE in adults and kiddos:
There are a variety of causes for SE that vary between populations. Acute processes account for the majority of cases of SE in adults, although a higher proportion of adults with SE have established epilepsy compared to the pediatric population. Some common acute and chronic causes of SE include the following:
Acute Causes of SE:
Infection/sepsis
Central Nervous System (CNS) infection
Metabolic disorder/electrolyte disturbance (hyponatremia, hypocalcemia, etc.)
Withdrawal (alcohol, benzodiazepine, antiepileptic)
Medication-induced/illicit substances
Brain injury (trauma, stroke, hypoxia from cardiac arrest)
Non-adherence to antiepileptics (low AED levels)
Hypertensive encephalopathy
Autoimmune encephalitis
Chronic Causes of SE:
Pre-existing epilepsy with breakthrough seizures
Abscess
CNS tumors
Remote traumatic brain injury (TBI)
Prior stroke
Other remote cause
Ok, now that we have that info down pat, it’s time for a quick side bar. You might already be thinking this question, so let’s just go ahead and address it.
What’s the difference between status epilepticus and febrile seizures in the pediatric population?
If you’re not already blown away by the epidemiology of SE (that was a bit of sarcasm…), this will surely do it. Febrile seizures, sometimes called fever-associated seizures, are actually the most common seizure condition in those aged 6 months to 5 years. As defined by the National Institute of Health (NIH), a febrile seizure is defined as a seizure accompanied by a fever (temperature at least 100.4*F) without evidence of intracranial infection or a defined cause of the seizure.
Essentially, the seizure is caused by a febrile illness not related to the CNS and does not meet criteria for other acute symptomatic seizures.
Febrile seizures are divided into 3 categories below:
Ahhh, so that’s how febrile seizures fit in with status epilepticus…
Pathophysiology of Status Epilepticus
The exact cellular mechanisms are unknown, but in general, seizure initiation results from an imbalance in excitatory (glutamate, sodium, calcium, substance P) and inhibitory (GABA, adenosine, potassium) neurotransmission. Excitatory pathways overcome inhibitory pathways.
Just like Lucy and Ethel, the brain can become overwhelmed in status epilepticus. Protective measures just can’t keep up! Of course being overwhelmed by chocolate is far more pleasant than being overwhelmed by proconvulsant stimuli… (Image)
After a single, brief, generalized tonic-clonic seizure, homeostatic inhibitory mechanisms activate to significantly elevate the seizure threshold (makes it harder to have a seizure) and restore normal transmission of neurotransmitters to prevent runaway seizure activity. When seizures occur in close succession, these normal homeostatic inhibitory mechanisms can fail, leading to severely elevated proconvulsant stimuli. This increased proconvulsant stimuli results in self-sustained seizure activity and possibly status epilepticus.
In general, SE can be classified into Generalized Convulsive Status Epilepticus (GCSE) and Non-convulsive Status Epilepticus (NCSE). Broadly speaking, patients with GCSE experience motor symptoms like rhythmic jerking of their extremities and increased muscle tone. Those with NCSE do not necessarily look like they are having seizures. In other words, there are no clinical findings, but seizure activity can be seen on an electroencephalogram (EEG).
You may be saying to yourself, “The patients without rhythmic jerking must be in a better shape, right!??” Well, not necessarily…
Even Homer didn’t know that status epilepticus could be non-convulsive. (Image)
The patients with NCSE often have a higher mortality rate because it’s harder to recognize that the patient is actually in SE!
Common Causes of Status Epilepticus
I know, I know… There is a pretty comprehensive list of acute and chronic causes of SE under the “Why” section above. But, it’s important to clarify common causes in the adult versus pediatric population.
In adults, acute events such as medication withdrawal, medication-induced seizures, and metabolic abnormalities serve as the most common etiologies. Stroke is also another major cause. In adults with prior existing epilepsy, low AED levels account for roughly 25% of SE episodes. (EEEK! Hello pharmacists! Let’s hop on that AED management!!)
In children, SE often presents as the first sign of epilepsy. Infections, such as CNS infections, and febrile status epilepticus are common causes of SE in children. Traumatic brain injury is another commonly cited cause of SE in children.
Drugs that Reduce the Seizure Threshold
If you are a pharmacist, pharmacy student, or pharmacy intern, this section should be VERY important to you, or at least I hope it is. There are many medications that reduce the seizure threshold by shifting the balance between inhibitory and excitatory pathways in the brain, leading to a seizure.
Opioid analgesics
Tramadol (FYI, can induce seizures even at therapeutic doses!)
Morphine
Antidepressants
Tricyclic antidepressants (TCAs), especially clomipramine and amitriptyline (N.B., Seizure rates reportedly as high as 2%!)
Bupropion (FYI, based on FDA reports, incidence of seizure activity ~ 0.6%)
SSRIs/SNRIs generally have no increased seizure risk at normal doses
Clozapine should generally be avoided in patients at risk/with a history of seizures; however, it’s reasonable to continue with close monitoring in most patients since clozapine is usually used as a treatment for refractory schizophrenia
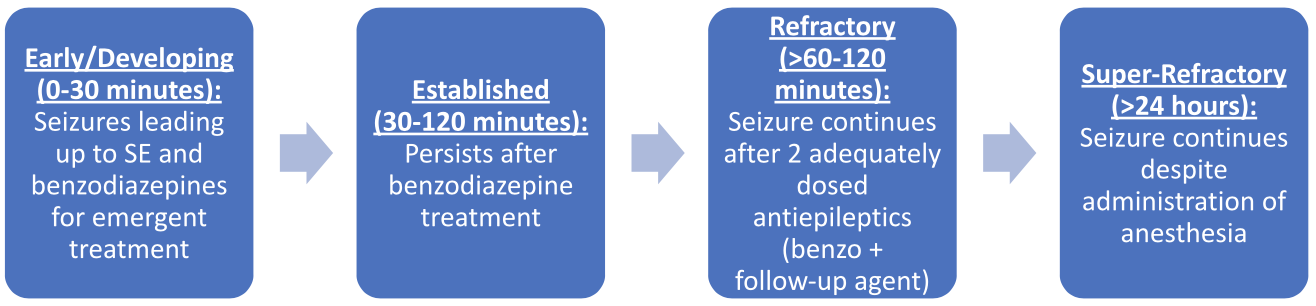
What are the 4 Stages of Status Epilepticus?
So, now that we have discussed the incidence of SE as well as the common causes in adults and children, we need to talk about what happens when someone is in SE. This will require talks of treatment, but let’s first describe the 4 stages of status epilepticus before getting into my favorite part… the medications.
(Image)
It’s important to start early treatment to prevent a patient from entering the later stages, which increase the chances of brain injury. Super-refractory SE is self-explanatory, so I applaud those who have established this name. It is difficult to treat and may require non-pharmacologic interventions.
How is Status Epilepticus Treated?
Early-Stage Status Epilepticus (Midazolam, Diazepam, and Lorazepam)
There are many guidelines and protocols that have been published on the management of SE, and all of them recommend the use of benzodiazepines as the drugs of choice for emergent initial treatment.
All of the benzodiazepines, discussed below, work by allosteric modulation of the GABA(a) receptor. This means they bind to the GABA(a) receptor at a location other than the site where the body’s natural ligand (GABA) binds. This facilitates the binding of GABA to its receptor, and in turn, slows down transmission of brain signals. (Remember, GABA is an inhibitory neurotransmitter!)
So, what benzodiazepines can we choose from?
Lorazepam, midazolam, and diazepam have been studied the most and are named in various guidelines, so we will focus on these agents. Here’s a quick intro to the nuts and bolts:
Now for the nuances of choosing an agent…
In general, administration of an intravenous (IV) benzodiazepine is preferred for SE due to its rapid onset and more predictable pharmacokinetics. However, if there is no IV access, my go-to is midazolam for IM administration. Think of the following to help you remember… MIDAZ-IM.
There was actually a study performed in adults and children experiencing seizures being treated by paramedics on route to the hospital, and IM midazolam was found to be noninferior to IV lorazepam. Therefore, in the prehospital setting and environments without easy IV access, IM midazolam may be superior to IV lorazepam and is the best choice. In this situation it reduces the time-to-treat period.
Of note, midazolam is the most lipophilic of the three benzodiazepines, but when given IM, it has a bit of a delayed onset of 5-15 minutes. Its duration is about 1-2 hours. An important note (yes…important…my preceptor has been asked this before), the IM dose of midazolam should not be repeated. Intravenous administration of midazolam is only used in refractory cases, not for initial treatment.
On to the next benzo. Lorazepam has emerged as the favorite due to its longer duration of action compared to diazepam, so in most cases, I would prefer lorazepam. It is the least lipophilic out of the three benzos, so its onset is slightly slower than diazepam, sitting at 5-10 minutes. However, to make up for that, its duration of action is the longest at 2-6 hours. This is because the overall time to cross into the CNS and eventually redistribute out is much longer than diazepam.
Only IV administration of lorazepam is indicated, so avoid IM lorazepam. Let me repeat, avoid IM lorazepam. The absorption is much too erratic and unpredictable, which is not desired in this inpatient scenario. Unlike midazolam, the IV lorazepam dose can be repeated x1 if seizure activity persists after 5 minutes.
If we were really concerned about choosing an agent with the fastest onset and we had IV access, diazepam would be the best choice. It has an onset of about 1 minute, which is the quickest of the three. However, it has a limited duration of action of 1-2 hours. This is because it undergoes rapid redistribution from the brain into the peripheral tissues. This may result in a higher likelihood of recurrent seizures.
Given this pharmacokinetic profile, most clinicians prefer lorazepam over diazepam. Nonetheless, you can repeat a dose after 5 minutes. An interesting pearl with diazepam centers around available formulations. It is available to be administered rectally, but its use is restricted to the pre-hospital setting when IV is not available.
Established Status Epilepticus (Levetiracetam, Valproate, Fosphenytoin)
The goal at this stage is two-fold:
Stop SE in patients who fail initial emergent treatment with benzodiazepines, and
Rapidly achieve therapeutic levels of an AED in patients responsive to emergent therapy.
Despite the existence of multiple randomized controlled trials, an evidence-based drug of choice in patients with established SE has not been established. There is not enough strong and consistent evidence, for instance, to distinguish differences in efficacy between IV valproate and IV phenytoin. The following drugs are the top preferred choices and should be considered: IV fosphenytoin/phenytoin, valproate sodium, and levetiracetam.
And now, if I can get a drum roll, please (here is where you start the drum roll) … Here is a breakdown of the most commonly used agents, including fosphenytoin and valproic acid, which are preferred by the Neurocritical Care Society guidelines:
The important question you’re probably, and hopefully, thinking to yourself is, “What drug do I choose?”
GREAT QUESTION! Well… as already hinted at, there is no drug that is clearly most effective. This was seen in the ESETT trial (2020), which compared the efficacy of levetiracetam, valproate, and fosphenytoin in children (less than 18 years old), adults (18-65 years old), and older adults (greater than 65 years old). The primary outcome was absence of clinically apparent seizures with improved consciousness without additional antiseizure medication at 1 hour from the start of the drug infusion.
In the end, there were no statistically significant differences detected in primary or safety outcomes between the drugs in each age group. Therefore, it may be reasonable to choose an agent that is safest and easiest to use for that particular patient. Because the devil’s in the details, let’s take a look at the deets of these three medications…
First up, fosphenytoin. In the absence of contraindications, I would choose fosphenytoin since it has been studied the most (much more than levetiracetam) and has arguably a better safety profile than valproate. It is also significantly cheaper than most of the new antiseizure medications.
Fosphenytoin is the water-soluble prodrug of phenytoin (for all things phenytoin, check out this previous tl;dr post). It blocks sodium (Na+) channels in the motor cortex, which prolongs the inactivation state of these channels, thereby controlling seizure activity. Fosphenytoin is rapidly converted 1:1 into phenytoin by plasma esterases, and it usually takes around 30-60 minutes for the active phenytoin to peak. Half-life is greatly variable due to saturable kinetics (it’s 95-99% protein bound!), and it can also depend on patient comorbidities.
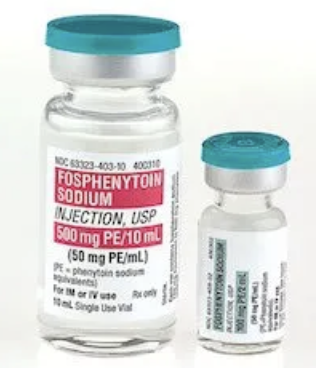
In terms of administration, fosphenytoin is preferred over phenytoin. This is because fosphenytoin can be administered faster, may be given IM as well as IV, is compatible in not only saline (like phenytoin) but also LR and dextrose, and is likely associated with fewer skin reactions compared to phenytoin. With regards to monitoring, fosphenytoin is similar to phenytoin (because really what you’re monitoring with fosphenytoin IS phenytoin…). Target total phenytoin levels range from 10-20 mg/L, and target free levels range from 1-2.5 mg/L.
See that? 50mg PE/mL. They’re not trying to be confusing…but they are. But let’s make them not. (Image)
On that note, let’s get something straight: phenytoin equivalents (or PE). This is something that we really need to use the Keep It Simple rule on. (Maybe we all just have PTSD from learning milliequivalents!)
The amount and concentration of fosphenytoin is always expressed in terms of “mg PE.” Again, this is because phenytoin is the active metabolite of fosphenytoin, so that’s really what we care about for therapeutic activity. Basically, 1.5 mg of fosphenytoin sodium is equivalent to 1 mg phenytoin sodium, and it is referred to as 1 mg phenytoin sodium equivalents (PE). So when you see a dose of fosphenytoin written as 250 mg PE, that’s equivalent to 250 mg of phenytoin.
You really shouldn’t ever receive an order for “250 mg of fosphenytoin” and then have to do proportions to figure out how many mg of phenytoin or PE that is… Friends don’t let friends prescribe fosphenytoin that way. If you DO receive an order like that, hop on the phone and clarify if the prescriber actually meant PE since that could substantially change the dose. #errorprone
Alright, that bit aside… Interestingly, fosphenytoin should not be used in pregnant patients and is contraindicated in certain cardiac conditions like 2nd or 3rd degree AV block, sinus bradycardia, SA block, and severe hypotension. In addition to this, phenytoin has been shown to cause other arrhythmias, so careful cardiac and respiratory monitoring is required.
Fosphenytoin fun done. On to the next med!
If drug-drug interactions or comorbidities are a concern, I would lean toward levetiracetam. It has simpler kinetics and a much better adverse effect and interaction profile than both valproate and phenytoin, which interact with CYP450 enzymes. Most interaction concerns are thus avoidable with levetiracetam.
To further support its safety profile, there are no absolute contraindications for levetiracetam use. Therefore, in emergent situations with limited information or history on the patient, this is a convenient choice.
This drug works in a somewhat unique way, selectively binding to synaptic vesicular protein SV 2 A, thus modifying glutamate release and facilitating GABA-ergic inhibitory neurotransmission. It works as fast as 5 minutes (peaks 5-30 minutes) and has a half life of 6-8 hours in adults and 5 hours in infants and children.
Okay, so something really cool to remember is that levetiracetam can be administered via IV push undiluted, in adults only. This can occur at the following rates:
Dose less than 3000 mg: Give over 5 minutes
Dose greater than or equal or 3000 mg: Give over 10 minutes
This is important in the SE clinical scenario since it prevents wasted time while waiting for compounded or diluted products to arrive. Lastly, although minimally metabolized, the half-life of levetiracetam can be increased in renal impairment, although this will likely not cause clinically significant toxicity in the short-term. Despite being an overwhelmingly safe drug, there is a risk of mood disturbance, agitation, and psychosis.
And now for the 3rd medication option of this section: IV valproic acid. Valproate is at least as effective as phenytoin in SE. It DOES NOT have a benign adverse effect profile, quite the opposite in fact!, especially concerning hepatotoxicity and hematologic abnormalities. It is preferred in patients with primary generalized epilepsies (especially children), and in these patients, I would recommend its use.
Valproate works by increasing GABA release and availability to neurons, while also inhibiting sodium channels. It is highly protein bound in general (80-90%) and also exhibits non-linear, concentration-dependent protein binding (increasing free fraction with increasing concentration). It has a half-life between 9-16 hours in adults, which is similar for infants and children greater than 2 months old. However, for neonates in the first week of life, the half-life is super long - like up to 45 hours!
Now, as mentioned previously, there are some safety risks associated with VPA, specifically being contraindicated in pregnancy and hepatic failure. It also interacts with other antiseizure medications, and it should not be used concomitantly with phenytoin/fosphenytoin. (Can you imagine the displacement interactions for 2 highly protein-bound AEDs?!?!? Eepers…!) Valproic acid may also cause hyperammonemia due to depletion of carnitine (necessary for its metabolism), which is a unique adverse effect that can lead to encephalopathy, increased intracranial pressure, seizures (ironic?), coma, and death.
On that note, monitor, monitor, monitor! In addition to the above clinical monitoring, there is also a role for therapeutic drug monitoring, with traditional target total VPA levels between 50-100 mg/dL and free levels 5-15 mg/dL. In SE, the target is slightly higher, at 80-150 mg/dL.
In summary, valproic acid is not my first choice, but it is a broad-spectrum agent and can be used in patients presenting with mixed seizure types.
Refractory Status Epilepticus Treatment (Intubation & Initiation of Propofol, Midazolam, or Pentobarbital Infusions)
If seizure activity has continued after emergent treatment with a benzodiazepine and urgent treatment with an IV AED, the refractory stage has started. 22-43% of patients enter this stage, which is not insignificant!
This persistence of seizure activity is commonly identified with continuous electroencephalography (cEEG) monitoring, which is that scary-looking contraption with electrodes that engulf a patient’s head. It is recommended to immediately start additional agents, particularly continuous infusions. At this point, if not already done, it is recommended to obtain a continuous EEG and secure the patient’s airway prior to induction of the sedative.
Since I am so nice, I have summarized the anesthetic drugs for refractory SE in the table below:
What do all these meds have in common? Well, all three either activate or agonize the GABA(a) receptor. Each should be titrated to cessation of electrographic seizures or burst suppression. Also, there is NOT strong evidence to support an optimal duration of treatment with IV anesthetics. But traditionally, once 24-48 hours of electrographic seizure control is maintained, the IV continuous infusion anesthetic is eventually withdrawn. Furthermore, non-sedating IV AEDs are often used concomitantly with these IV anesthetic infusions.
So, the next question to ask yourself…and I hope you are picking up a pattern here… When would one agent (midazolam, propofol, or pentobarbital) be preferred over another?
There currently is not enough evidence to label one agent preferred. Or in other words, there is not a good, evidence-guided answer. That being said, the following info may help guide your decision-making.
Midazolam is a fine choice. It will likely cause less hypotension than either propofol or pentobarbital. So, in patients with severe shock where hemodynamic status is a concern, midazolam may be preferred. It works within 2-3 minutes and has a half-life of 4-6 hours. However, over time it accumulates, and the half-life may be extended.
No, not that kind of tacky… (Image)
Additionally, ongoing use may result in tachyphylaxis (you might need to sound that one out…tacky-fih-lax-us!). Tachyphylaxis refers to the reduction in the effectiveness of a drug as its use is prolonged. So, if infusion is necessary more than 48-72 hours, midazolam effectiveness may start to wean. The bottom line is that midazolam is still a reasonable first choice.
Next we have propofol. Its main advantage over midazolam is that propofol is more easily titratable, with a quicker offset than midazolam. It works within 1-2 minutes, and has a distribution half-life of 2-4 minutes and elimination half-life of 30-60 minutes. So, if there is a need for quicker recovery from sedation, propofol may be preferred over midazolam. In practical terms, extubation will likely occur quicker with propofol compared to midazolam.
What makes propofol use concerning, you may ask? If high doses of sedation are required for seizure cessation, propofol may be a concern due to its hypotensive effects and risk of PRIS (which is more common at high doses, e.g., greater than 5 mg/kg/hr for more than 2 days). Hypotension from propofol may be more prominent in pediatrics. It also should be noted that the hypotensive effects of propofol are due to dose-related vasodilation, so higher doses may be a concern.
There are a number of monitoring recommendations, including serial CPK and triglycerides. Elevations in CPK can put patients at risk for rhabdomyolysis.
And finally, there’s pentobarbital. Pentobarbital is more commonly used in super-refractory states and may be reasonable to use in situations where re-dosing midazolam or propofol is unsafe. I would try to avoid this agent due to numerous side effects (including hypotension, respiratory depression, and IV propylene glycol toxicity), drug interactions, and a sluggish half-life.
It works within 3-5 minutes but has a half-life of 15-50 hours. This can put patients in a persistent coma, drastically delaying extubation and prolonging ICU stay. At high doses, patients can completely lose neurological function. Yep, that’s SCARY!
If either midazolam or propofol is deemed ineffective when being used as monotherapy, the two are commonly used together as combination therapy in refractory SE.
The tl;dr of Status Epilepticus (SE)
Status epilepticus (SE) is a medical emergency defined as persistent seizure activity for at least 5 minutes or the occurrence of multiple seizures without regaining normal mental status in-between. The occurrence of SE is most common in young children less than 4 years old and older adults greater than 65 years old, with overall mortality being higher in adults than children.
Febrile seizures are characterized as seizure activity accompanied by febrile illness not related to a brain infection. They are more common than febrile status epilepticus and usually less serious. Febrile seizures can develop into febrile status epilepticus if they persist more than 30 minutes.
There are multiple acute and chronic causes of SE in adults and pediatrics. In pediatrics, infections are a common cause of SE.
SE can be broken into multiple stages depending on how responsive a patient is to therapy. They are as follows: developing/early-stage, established, refractory, and super-refractory. Benzodiazepines are first-line for initial emergent therapy, with IV AEDs (VPA, levetiracetam, and fosphenytoin) used for established disease, and IV anesthetics (midazolam, propofol, pentobarbital) in refractory cases. Choice of agent may depend on multiple factors including comorbidities, concomitant medications, hemodynamic status, and cost.