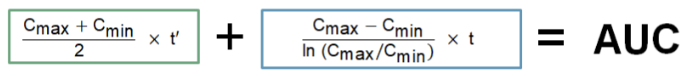The Complete (but Practical) Guide to Dosing Vancomycin Based on AUC/MIC Targets
Steph’s Note: I officially declare 2022 the year of fulfilling long overdue posts on tl;dr! First, it was vasopressors, then DKA, then forays into the retail and industry worlds, and most recently, delving into beta blocker pharmacology. We are crossing off wish list items left and right!
This week, we’re checking off THE #1 item on your list for Santa. Cue the drumroll. (I can hardly believe I’m saying this because I’m just so tickled pink excited. You know I love vancomycin and pharmacokinetics, #nerdalert.)
We are returning to the world of vancomycin and FINALLY breaking down the new kid on the block: AUC/MIC dosing. Please give Drs. Dev Chatterji and Annie Jeon of Inova Fairfax Medical Campus a huge round of applause for tackling this topic for us and explaining it so stinkin’ well. (If you don’t know Dr. Chatterji yet, you should definitely check out his previous posts on pneumonia and UTIs.)
Let the games begin!
True story. (Image)
Just when you thought hospital pharmacy practice couldn’t get any more complicated… If you’re an avid tl;dr pharmacy reader, you have likely bookmarked and routinely utilize Steph’s Complete (but Practical) Guide to Vancomycin Dosing. You know us pharmacists….we love to dose us some vanco!
(Image)
And after reading it half a dozen times, you likely thought you had the vancomycin dosing process down, right? You knew what levels to target based on indication. You felt impactful during rounds as you made those dose adjustments. You’ve got your note template all set to input notes in the chart.
Well, I’ve got a ‘curve’ ball (no pun intended) coming your way… Get ready to learn a whole new set of recommendations that basically revamps the theory of targeting that ideal trough level. That’s right, they sneaked all new vancomycin guidelines into the mix just as we got busy with COVID in 2020.
But wait! Don’t erase all the expertise you gained from that wonderful vancomycin post…
Parts 1 and 2 that review the PK/PD of vancomycin obviously have not changed. Not to mention, the new AUC/MIC dosing process technically applies to serious invasive MRSA infections (i.e., bacteremia, sepsis, infective endocarditis, pneumonia, osteomyelitis, and/or meningitis). The data is lacking in other types of infections as well as infections due to other pathogens you may be utilizing vancomycin for.
Therefore, extrapolating this AUC/MIC system to the use of vancomycin in other, non-serious infections (e.g., non-bacteremic skin and skin structure infections, UTIs) and in the treatment of infections due to other bugs (e.g., MSSA, coagulase-negative staphylococci, etc.) should be done cautiously.
So caveats aside, what is this AUC nonsense?
What is the Vancomycin AUC?
(Image)
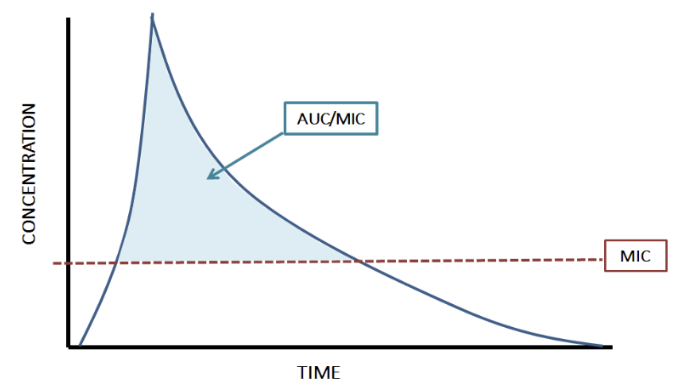
Well if you happen to remember from The Complete (but Practical) Guide to Vancomycin Dosing, vancomycin is neither concentration-dependent nor time-dependent. Rather, it’s a combination of both. As Steph previously explained, the killing action of vancomycin is dependent on the concentration of the medication and the amount of time that the concentrations are maintained. This makes vancomycin “AUC-dependent,” which stands for Area Under the Curve.
So AUC was ALWAYS the true PK target. We’ve just been using troughs as a simple surrogate because they’ve been associated with AUC. However, as AUC calculations were evaluated in more depth in recent years, we saw that troughs weren’t always perfectly correlating with AUC.
Therefore, let’s skip the surrogate since it turns out it’s not a great one anyway. It makes more sense for us to use the AUC/MIC as the true marker for efficacy, rather than the trough target of 10-20 mcg/mL.
The AUC is the whole area under the blue curve line that accounts for both time and concentration. It includes both the blue and white space above and below the MIC line. (Kind of like basic geometry, area = length x width - or in this case, time x concentration.) However, because we’re shooting for efficacy against an infection, we really only care about the AUC that is above the MIC of the bacteria, which is why nothing lower than the MIC line is shaded blue. So AUC/MIC is the blue-shaded area, and this is what we’re targeting with the new dosing recommendations.
Btw, these figures are quite the upgrade from the original vancomycin post, wouldn’t you agree? Thank you, Annie Jeon!
Vancomycin AUC/MIC Dosing Versus Traditional Troughs
Maybe you’re asking – why haven’t we been using this system in the first place?
Well, it’s been impractical to try to calculate the AUC in the clinical setting. So in the name of the KISS rule (Keep It Simple, Silly), we used troughs. Plus, studies suggested that a trough of roughly 15-20 mcg/mL correlated with an AUC/MIC of 400-600 mg·h/L, so why work harder if we didn’t have to…
BUT newer data shows that this relationship is not as strong as we thought it to be. (Oops.)
Some studies suggest that troughs of 15 mcg/mL are not even necessary to reach an AUC of 400 mg·h/L, meaning that people with good, functioning kidneys have had therapeutic benefits with troughs <15 mcg/mL. This may suggest that maybe we should be targeting more conservative troughs since trough levels > 15 mcg/mL can increase the risk for toxicity compared to trough levels < 15 mcg/mL.
(Image)
And we as a pharmacy profession will just have to admit we slightly misspoke… Vancomycin levels in the upper end of the 15-20 mcg/ml range ARE associated kidney damage.
As many of us already know, the biggest concern that comes with vancomycin is AKI (acute kidney injury), defined as:
Increase in serum creatinine (SCr) level of ≥ 0.5 mg/dL OR
50% increase in SCr from baseline in consecutive daily readings OR
Decrease in CrCl of 50% from baseline on 2 consecutive days in the absence of an alternative explanation.
AKI is associated with higher trough values and higher AUCs. That makes sense – the more drug in your system, the more likely that it could potentially do harm. And we want to save those kidneys! What’s even worse is when patients have pre-existing factors that put them at even MORE risk: increased weight, pre-existing renal dysfunction, critical illness, or concurrent nephrotoxic agents (e.g., aminoglycosides, loop diuretics, amphotericin B, IV contrast dye, vasopressors, etc., etc.).
Newer data suggests that AUC-guided vancomycin dosing may be associated with reduced risks for vancomycin-induced AKI. In fact, in two 2017 publications, AUC-guided dosing was found to be independently associated with significantly decreased rates of AKI.
So what are these magic numbers you may ask?
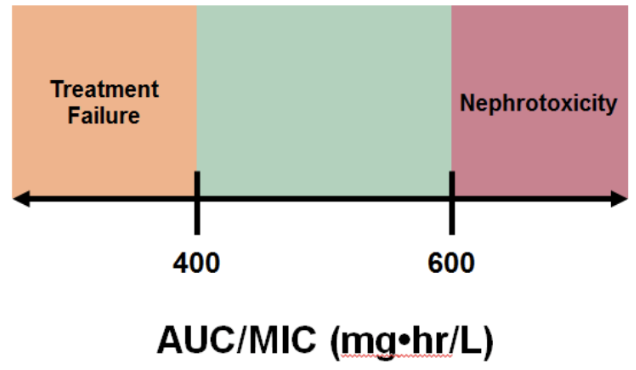
Based on current data, your goal should be to aim for an AUC/MIC somewhere between 400 and 600 mg·h/L (assuming MIC = 1 mg/L). This is a fairly narrow therapeutic window. AUC/MIC > 400 mg·h/L is to maintain efficacy, meaning you want it to work against that infection. If you aim < 400 mg·h/L, then there’s a higher risk of not eradicating the infection, while also inducing resistance to the drug.
Conversely, AUC/MIC < 600 mg·h/L is to maintain safety, which, in this case, means you want to save your kidneys from an acute injury. If you aim > 600 mg·h/L, you risk nephrotoxicity and possibly even permanent kidney damage.
How to Calculate the Vancomycin AUC
Got it. So how do we calculate an AUC if it’s so impractical?
Remember the big picture…. Pharmacokinetics in its broadest sense characterizes drug exposure and concentration per unit time.
So you have two main options for calculating an AUC:
First-order PK equations
Bayesian derived calculations (using software)
Option #1: First-order Vancomycin PK Analytic Equations
What you need: Two timed, steady-state serum vancomycin concentrations.
Preferred data points: Near steady-state, post-distributional peak (~1-2 hours after end of infusion) plus a trough concentration within the same dosing interval.
This is routinely referred to as the trapezoidal method. These two articles do a decent job explaining the trapezoidal method in more detail for those that may be interested. But ok, here’s the ‘long-hand’ way to calculate an AUC using first order PK (not exactly a tl;dr approach, but comprehensive nonetheless).
Equation for calculating the AUC:
What you do:
Visualize HOW you get those two numbers in the above equation,
Obtain those two timed, steady-state vancomycin concentrations mentioned above and start doing some math.
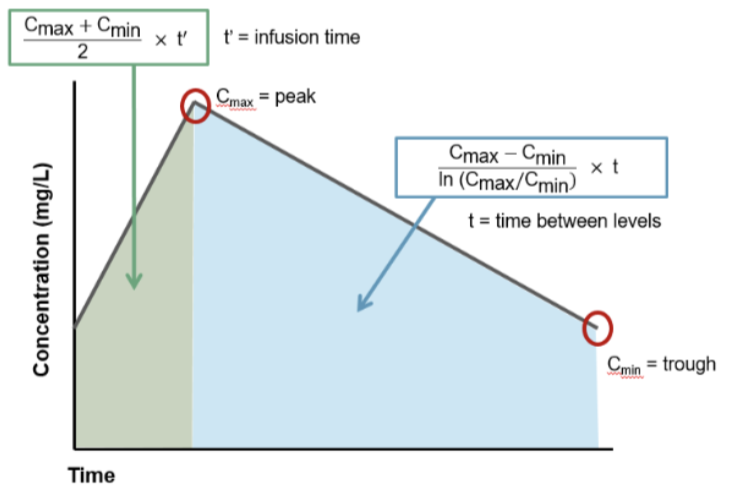
For the tl;dr version of the trapezoidal rule, we’re basically finding the area of 2 trapezoids that make up the vancomycin AUC. In this figure, there’s a green trapezoid and a blue trapezoid. If we can find the areas of those 2 shapes and add them together, voila, we have the AUC.
The green trapezoid is the easy one because it follows linear geometry, meaning the rise of the concentration values follows a linear slope. Infusion rate doesn’t change, right? You set the pump to x mL/h, and that’s how fast it infuses. So that trapezoid is just a normal, straight-lines-on-all-sides trapezoid. For it, you can go back to basic geometry yet again…
Area of a trapezoid = 1/2 x (base 1 + base 2) x h. Applying this formula to our vancomycin linear green trapezoid, the “bases” of the trapezoid are the concentrations, Cmin and Cmax, because those numbers tell us the lengths of the parallel sides of the trapezoid. So base 1 of the green trapezoid is actually Cmin, and base 2 of the green trapezoid is the Cmax. The height (h) of the trapezoid, aka the distance between the parallel sides, is the infusion time. Now does that make that formula above boxed in green a little more sensible? It’s just the area of a trapezoid using vancomycin terms!
Unfortunately, the area of the blue trapezoid is trickier. That’s because it follows a logarithmic curve, meaning the line between Cmax and Cmin is not straight (even though we’ve drawn it that way for simplicity’s sake). So additional derivations are necessary in order to determine the k (or slope) of that line, which can then be used to find the area of that section. For all you math-happy people who want the details, check out the proof behind the equation in the blue box here!
Unless you work in a perfect hospital in a perfect world, you aren’t actually going to have measured serum concentrations that are exact peaks and troughs. Instead, if you’re lucky, you should have 2 measures that are close to those…but lie on adjacent points of the time/concentration curve. (If you’re unlucky, the levels were drawn at completely incorrect times…or during the vancomycin infusion…or on a totally different day than ordered! Oh, the joys of checking levels!)
Assuming you have two concentrations within the same dosing interval, you can use those to obtain your patient specific Ke, which you can then use to back calculate your C max and C min. If you need a refresher on how to do this, check out this post. Plus, the figure below is for you visual folks.
Now back to the steps of this process.
3. Once you have all these values, then you can calculate the area in the green shaded space and add it to the area in the blue shaded space. And that equals your AUC!
Advantages of the First Order PK Strategy for Vancomycin AUC
Relies on fewer assumptions than the Bayesian approach (Option #2, hold tight).
First-order PK equations are familiar to most clinicians. Once the AUC over 24 hours is calculated, the total daily dose can just be adjusted based on target goals.
While it may seem like a lot of fancy math, you can build a calculator with a program (such as MS Excel) so you’re not doing all that long hand math on paper. This is a pretty cost effective way to do it.
Limitations of the First Order PK Strategy for Vancomycin AUC
Only provides a snapshot for a given period of time (less adaptive than the Bayesian method).
Cannot adapt to physiological changes that occur after samples are drawn (e.g., renal function changes).
Difficult to equate 24-hour AUC when patients are on multiple dosing regimens within a day.
Samples should be drawn when steady-state concentrations are achieved in order to be more accurate. Obtaining two accurately timed levels is difficult from a workflow perspective and relies heavily on nursing/lab tech staff.
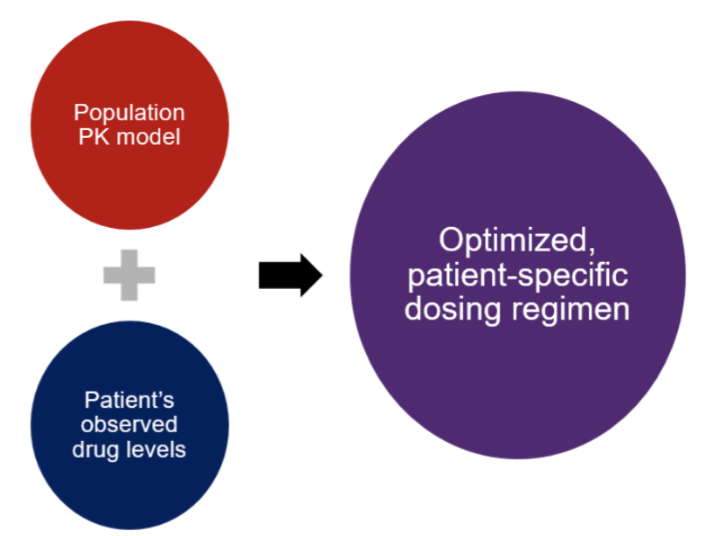
Option #2: Bayesian-derived Vancomycin AUC Monitoring
What you need: One level at a minimum. Potentially two vancomycin concentrations for select patient populations to make your AUC estimate more accurate.
How it works: Many institutions now have adopted the Bayesian software. This is a prediction tool based on drug behavior in various patient populations with their respective PK parameters (e.g., volume of distribution, clearance). It uses data gathered from a well-developed vancomycin population PK model to calculate an optimized dose based on the individual patient’s specific characteristics.
Advantages of the Bayesian Vancomycin AUC Strategy
You don’t need steady-state lab values (which typically occur after 3-4 consistent doses) . Therefore, samples can be drawn in the first 24-48 hours to drive subsequent dosing regimens.
Provides the ability to factor in creatinine clearance, which can fluctuate based on patient’s status.
Can theoretically work with only one trough value.
Limitations of the Bayesian Vancomycin AUC Strategy
Although a single random level may be used for calculations, it may not be accurate across all patient populations.
Calculations are based on a pool of prior vancomycin patients, and they make many assumptions.
It’s expensive to implement in an institution!
Do we still load vancomycin in the AUC/MIC dosing world?
Yes! Loading doses are still recommended in patients who are critically ill or in the ICU, require dialysis or renal replacement therapy, or are receiving continuous infusion therapy with vancomycin. Loading doses are used to achieve target ranges of serum vancomycin concentrations faster and reduce the risk of getting lower serum concentrations in the first few days of therapy.
The recommended loading dose is ~25 mg/kg based on actual body weight with a maximum dose ~3000 mg. (Remember, this may depend on institution-specific practices. For example, some institutions cap their vancomycin doses at 2000 mg instead.)
The tl;dr of AUC/MIC Vancomycin Dosing
Well, you’ve survived this basic intro to AUC/MIC targeted dosing strategies for vancomycin!
Key overall takeaway points…
In order to optimize and maintain efficacy as well as decrease toxicity of vancomycin, the updated ASHP/IDSA guidelines for the therapeutic monitoring of vancomycin recommend changing the practice of vancomycin monitoring from targeting vancomycin trough levels to targeting overall vancomycin exposure. This is measured by the area under the 24 hours concentration curve, or AUC.
A vancomycin AUC of 400-600 mg·h/L range is recommended for most severe infections due to Staphylococcus aureus. While many clinicians/institutions are targeting the same AUC range whenever vancomycin therapy is employed, we have to remember that most of the data and recommendations come from patients with serious Staphylococcus aureus infections.
With that said, the trough-based targets we have been using for years were also meant for Staphylococcus aureus infections. So personally, I feel like targeting the AUC range recommended should be just fine for those as well.
Some key things to remember about AUC-based dosing and how it will change practice…
An AUC is a calculated value using high level pharmacokinetic analysis. Level monitoring does not go away! You still need vancomycin levels to calculate the AUC. Its just that depending on the method you use, the timing of the levels may be different than what you are typically used to.
Two primary options recommended to calculate the AUC are first order PK calculations (i.e., the trapezoidal method) and Bayesian software programs.
AUC-based vancomycin dosing generally results in lower doses of vancomycin. Trough levels that may have historically been interpreted as low may actually be therapeutic when using AUC-based calculations. Vancomycin levels should not be taken at face value anymore since AUC is a calculated value. Also, you may see more variety in the timing of levels.
(Image)