Pharmacology 101: An Overview of Beta Blockers
Steph’s Note: This week, we’re returning to our pharmacy roots with a new Pharmacology 101 topic! Building on our previous posts about statins, thiazide diuretics, ACE-inhibitors, and vasopressors, we’re LOL’ing about the inherently funny -olols, aka the beta blockers. (Wow, that was a lot of cheese, wasn’t it? I almost couldn’t get through typing the sentence, that’s how much I cringed. But c’mon, the lol was just too much to resist.)
Bringing you this pharmacology knowledge drop is Ahlam Ayyad, who is a clinical assistant professor at Xavier University of Louisiana College of Pharmacy. She received her Doctor of Pharmacy Degree from Xavier University of Louisiana College of Pharmacy and completed her post graduate residency training at East Jefferson General Hospital. Her current practice site is at the Southeast Louisiana Veterans Health Care System with the internal medicine team. Her current interests include nephrology, psychiatry, and infectious diseases.
(Image)
Beta Receptors and the Nervous System
Well actually, before we get to the medications, let’s start with some basics because ain’t nobody gonna understand a beta blocker without first grasping beta receptors. And ain’t nobody gonna get a beta receptor without understanding the nervous system.
So how do beta receptors fit in the overall nervous system, and where are they located?
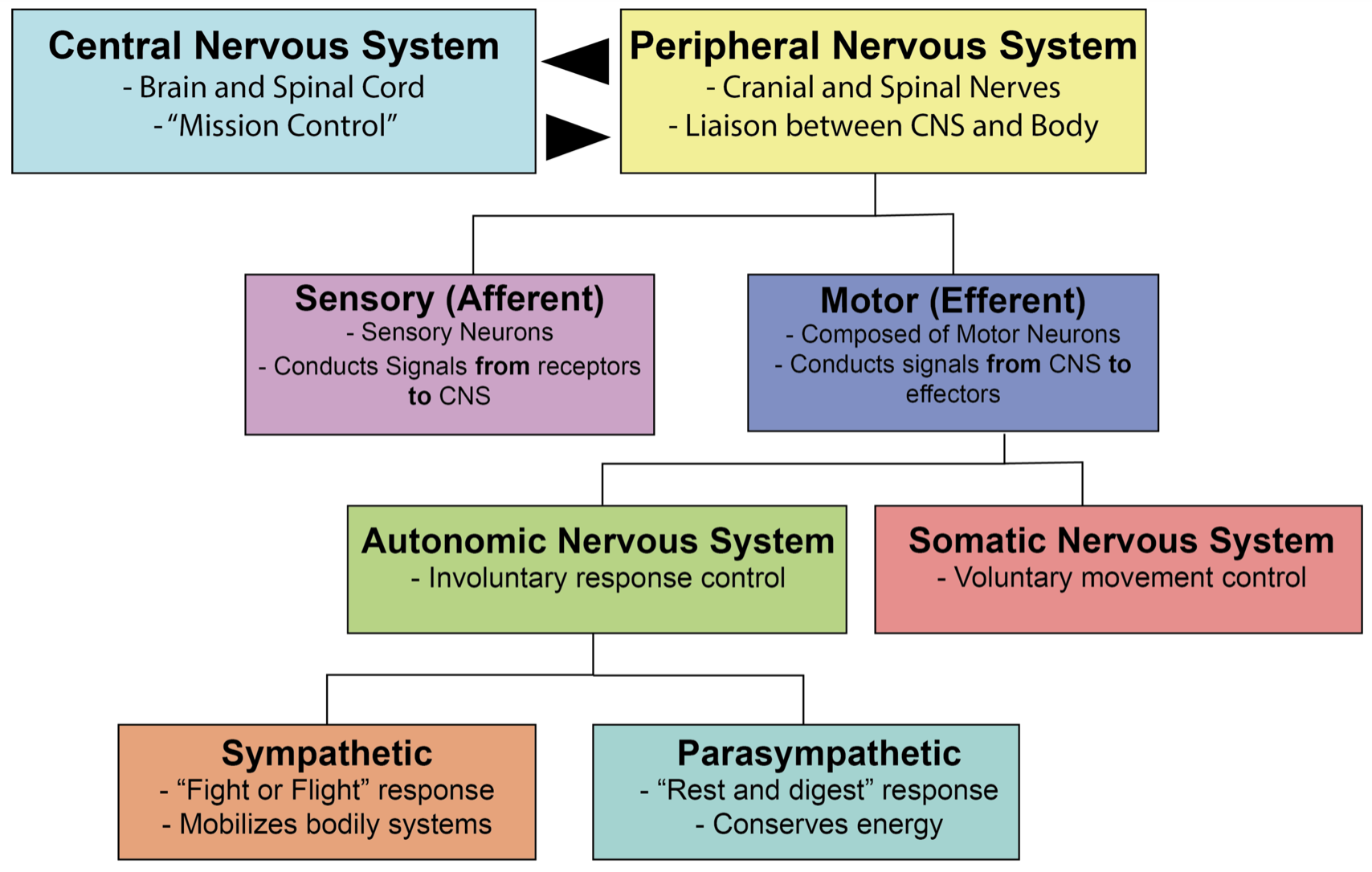
Beta adrenergic receptors are part of the autonomic nervous system (ANS). Throwback to anatomy/physiology 101 here! Remember that the body’s nervous system is divided into the central nervous system (CNS) and peripheral nervous system. The peripheral nervous system is further subdivided into the somatic and autonomic nervous systems. The somatic system is responsible for enervating the muscles you voluntarily control. On the other hand, the autonomic nervous system (ANS) controls your involuntary bodily processes and is broken down into the sympathetic (“fight or flight”) and parasympathetic (“rest and digest”) nervous systems, as well as the enteric nervous system.
Do you feel a bit like “the foot bone is connected to the…leg bone. The leg bone is connected to the…hip bone…”? Because I do. So let’s have a visual summary moment:
(Image)
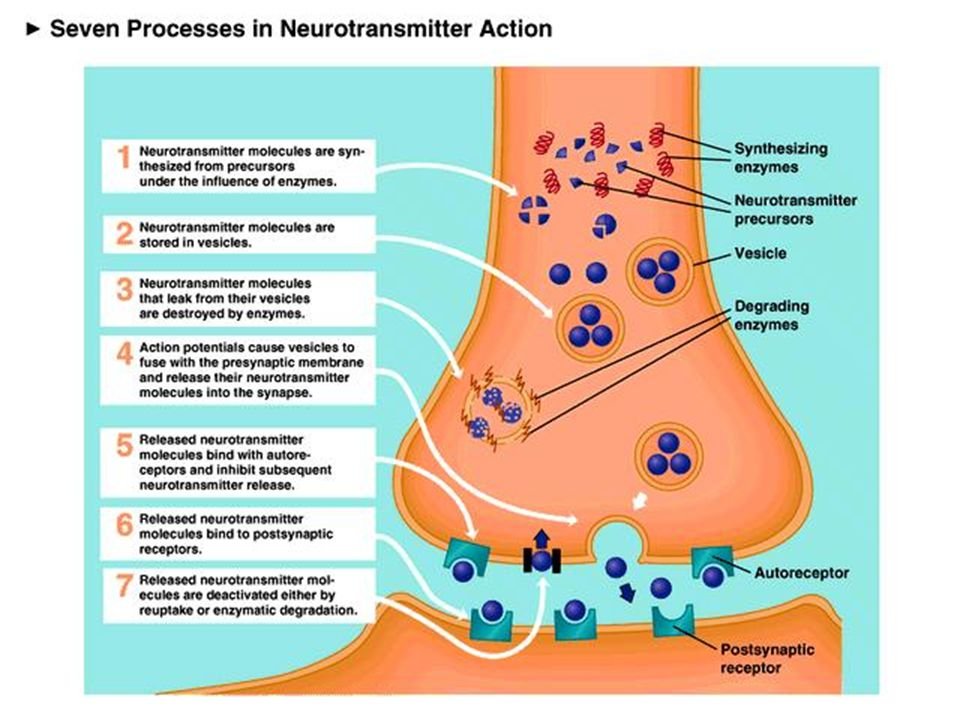
Like the other portions of the overall nervous system, the ANS relies on a network of neurons to send electrical and chemical signals. Nerve cells send chemical messages to the cells they enervate (cardiomyocytes, smooth muscle in the lungs, etc) via neurotransmitter molecules, which include the sympathetic catecholamines - norepinephrine, epinephrine, and dopamine. These neurotransmitters travel across the synaptic cleft to the post-synaptic cell membrane and bind to their intended receptors to mediate processes.
Again, for you visual folks, here’s a pictorial summary of the happenings at the synaptic cleft:
(Image)
In the case of norepinephrine, it binds to both alpha and beta adrenergic receptors on the post-synaptic membrane of the effector cell. Alpha and beta adrenergic receptors belong to a super family of receptors known as G-protein coupled receptors (or GPCRs). GPCRs are large, 7 transmembrane-domain molecules that, when agonized by their ligands, spur a cascade of activities to create downstream effects.
Sounds vague, right? A receptor that has a downstream effect when agonized. Grrrrreat.
Well, it’s kind of a vague statement because GPCRs are so diverse!! We don’t fully understand the ones that have already been isolated, and there are so many more to study.
To add to this, not all GPCRs function the same way. GPCRs can be subdivided into (at least for now) 3 categories: Gs, Gi, and Gq. Gs stands for stimulatory, Gi is inhibitory, and Gq is… I don’t know. It’s just Gq, trust me on this.
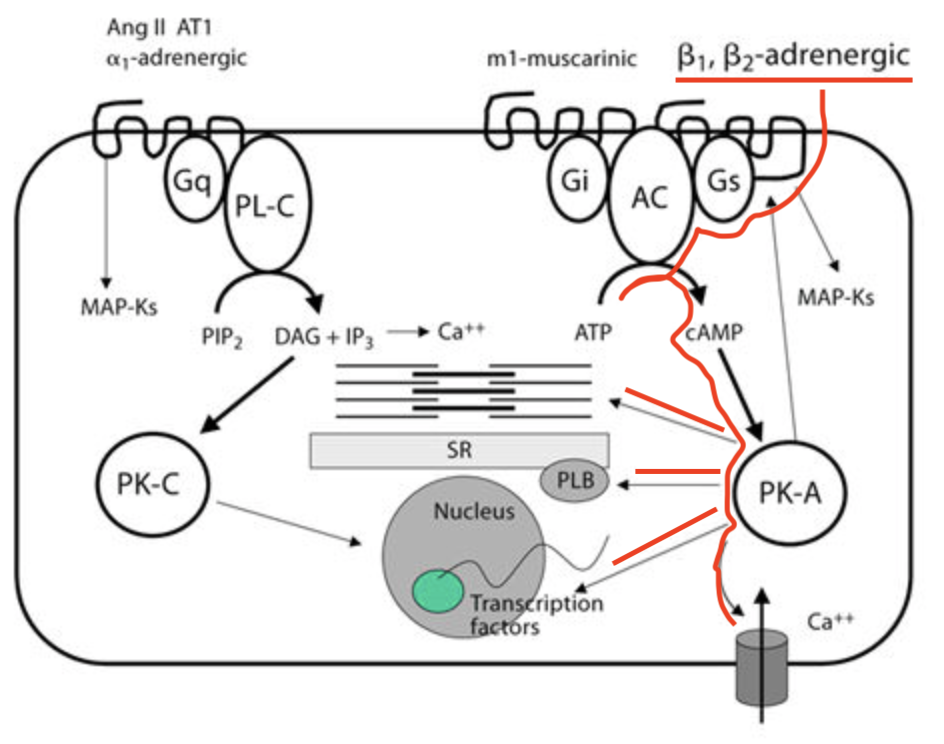
Regardless of what each G subtype stands for, it’s important to know that agonization of both Gs and Gi types of GPCRs leads to changes in adenylyl cyclase, which is the enzyme responsible for converting the ATP powerhouse molecule to a very important second messenger - cyclic AMP (cAMP). In the Gs pathway, newly-synthesized cAMP travels and binds to protein kinase A (PKA). PKA phosphorylates a wide variety of protein targets, leading to the ultimate end effect of the GPCR. This can include activities like calcium influx into the cardiac myocyte to produce contraction.
Focus on the portion highlighted in red, which represents the beta-adrenergic GPCR pathway. Important notes… See the 7 transmembrane domains of the GPCR (the black squiggly line through the outer cell line). These molecules are LARGE. Also, note that the beta-adrenergic receptor is coupled to Gs, which is the stimulatory pathway. When bound by its ligand (e.g., norepinephrine), this Gs GPCR path activates adenylyl cyclase (AC) to increase production of cAMP from ATP. Then cAMP binds to PKA, which kicks off any number of end results, such as calcium influx, up regulation of transcription factors, or changes to the sarcoplasmic reticulum and the contractile mechanism of the cell. Pretty cool, right? (Image)
FYI, Gq GPCRs do not follow the same adenylyl cyclase-related pathways. They have different players on the field in their cascades, which we won’t get into here. Not only because this is tl;dr, but because the receptors we care about for this post - the beta adrenergic receptors - are coupled to Gs GPCRs. So we’re going to keep it simple and not go on a Gq tangent.
Ok, so let’s take time for a quick recap. We’re talking about beta adrenergic receptors, which are Gs GPCRs located on post-synaptic effector cells. These beta adrenergic receptors bind to the neurotransmitters norepinephrine and epinephrine, which leads to upregulation of adenylyl cyclase and its product cAMP. This increased cAMP then binds to PKA, which executes the end process via phosphorylation.
So now you may (rightly so) be asking yourself, “What are these effector cells for beta receptors, and what are the end processes?”
Ahhh, more beta receptor meat!
So there are 3 known types of beta adrenergic receptors (although there’s apparently debate about a possible fourth?!). These are beta-1, beta-2, and beta-3. Creative, I know. The effector cells vary by receptor subtype, as does the end process mediated by the GPCR.
(Image)
Beta-1 adrenergic receptors are located primarily in the heart, specifically in the sinoatrial (SA) node, the atrioventricular (AV) node, and the ventricular muscle. So those are the aforementioned effector cells for the beta-1 receptors. To keep this straight, think of your better 1, your better half is in your heart.
Aw, how cute. I know this is not on your mind right now, but you will definitely remember it forever with that cheesy analogy.
Beta-2 receptors are in many organ systems, most notably the lungs and vasculature but also the GI tract, the liver, and urinary musculature. For simplicity’s sake, think of 2 lungs = beta-2 receptor. Agonization of these receptors contributes to metabolic activity (such as glycogenolysis in the liver) as well as smooth muscle relaxation, which is the usual pharmacologic target.
Beta-3 receptors are located in adipose tissue and contribute to lipolysis (the breakdown of fat). These are less clinically relevant at this point. However, there are also beta-3 receptors located in the detrusor muscle of the bladder, and these are targets for agonist overactive bladder medications like mirabegron. But those meds are out of the scope of this beta blocker post!
So what are the end effects of beta-1 and beta-2 receptors?
(Image)
Beta-1 receptors are part of the normal function of the sympathetic nervous system, also known as the ‘fight or flight’ system. It is the body’s way of recruiting extraordinary function in a short amount of time. By increasing heart rate and contractility, for example, the blood circulates more throughout the body, allowing a person to run faster, jump higher, and accomplish things not typically within their physiologic capacity for a short duration. It is a primary survival mechanism. Think of watching a scary movie or your reaction to a surprise party!
Beta-2 receptor agonism causes smooth muscle relaxation. For the beta-2 receptors located in blood vessels, this equates to vasodilation, leading to reduced peripheral vascular resistance and arterial blood pressure. Think of the pressure that builds up when you pinch a water hose and then the subsequent relaxation and release of pressure when you let go. This is similar to the dilation and relaxation that occurs in your blood vessels when beta-2 receptors are activated.
How do beta blockers work?
Ahhh, now that we’ve refreshed on the physiology, we get to the pharmacology!
Beta-blockers, aka beta-adrenergic receptor antagonists, do exactly what their name states. They compete with the beta-receptors’ endogenous ligands (those sympathetic catecholamines, especially norepinephrine and epinephrine) to prevent them from binding to their receptors, which prevents the neurotransmitters’ downstream effects.
Medications that selectively antagonize the beta-1 receptor lead to reduced heart rate, contractility, and blood pressure. Non-selective beta blockers refer to medications that antagonize both beta-1 and beta-2 receptors to varying degrees with net effects that also amount to lower heart rate, decreased contractility, and lower blood pressure.
(Confusing, I know… why would medications that block beta-2 receptors lead to lower blood pressure when it’s beta-2 agonism that produces smooth muscle relaxation? Well, the clinical effects from the beta-1 antagonism outweigh the effects from beta-2 antagonism for our non-selective beta blocker medications, not to mention some of the non-selective medications also have alpha receptor blocking properties as well, which certainly helps with lowering blood pressure!)
Non-selective beta blockade also refers to effects outside of the cardiac system - these medications may act on beta receptors in the GI tract and lungs (in addition to the heart and blood vessels), leading to slowed breathing, tremor prevention, and assistance with performance anxiety.
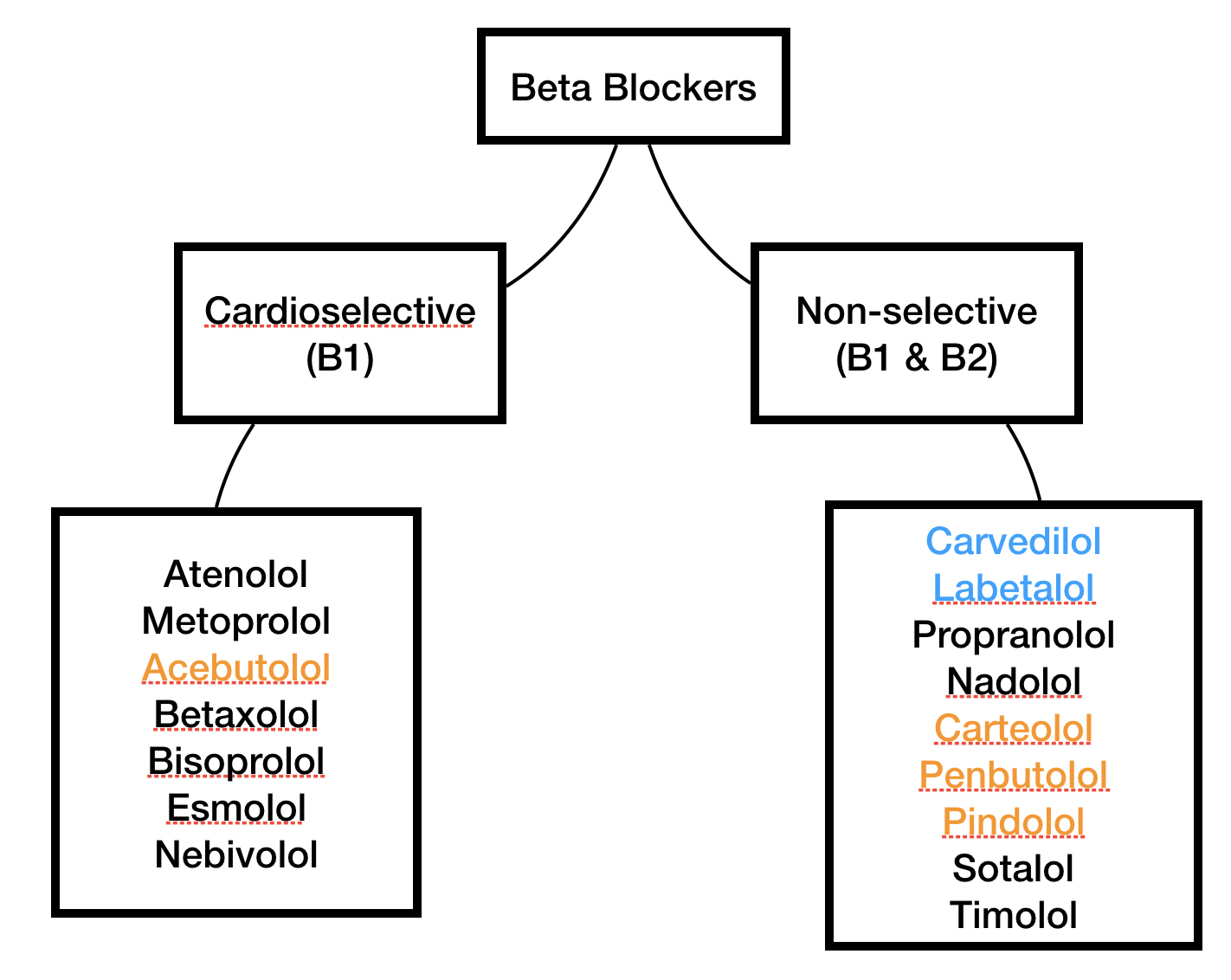
Orange medications have ISA. Blue medications have some alpha-adrenergic blocking properties as well as antagonizing both beta-1 and beta-2.
There are also some medications that simultaneously block the beta receptor while partially agonizing it! This partial agonist property is known as intrinsic sympathomimetic activity (ISA), and it can be useful for mitigating some of the adverse effects of beta blocking medications since the receptor’s effects aren’t 100% shut down.
So now that we’ve described the beta blocker medication class in terms of beta selectivity and ISA, please check out the visual summary to the right and meet the members of the beta blocker family.
(Image)
Knowing these concepts is super important to understanding indications for each medication. I know… as if we needed more things to remember.
When are beta blockers used?
I mean, honestly, it might be easier to talk about when beta blockers are not used… Only slightly kidding.
But really, beta blockers are cornerstone cardiac medications and are used in a plethora of conditions, including angina, atrial fibrillation and other arrhythmias, hypertension (although not first line without comorbidities!), myocardial infarction, hypertrophic cardiomyopathy, and congestive heart failure. Then, there are the non-cardiac indications, such as tremor, performance anxiety, migraine management, cirrhosis and associated variceal hemorrhage prophylaxis, thyrotoxicosis, and pheochromocytoma, just to name a few!
(Image)
So don’t put our baby beta blockers in a corner, eh? They’re capable of a LOT, even outside the world of cardiology!
That being said, as we’ve already touched on, not all beta blockers are created equally. Just because one medication helps with migraines doesn’t mean you can just substitute any other beta blocker for the same indication. Be sure to check indications and properties before choosing one of these agents.
How are beta blockers monitored?
You can likely surmise the answers here based on our extensive discussion of mechanism. But for the sake of completeness:
Blood pressure
Heart rate
Potentially an EKG depending on what the beta blocker is being used for…
Clinical Pearls for the Beta Blocker Medication Class
Beta blockers should not be abruptly stopped because the body gets used to the homeostasis caused from consistently taking them. If abruptly discontinued, a patient’s symptoms can worsen due to the subsequent increase in heart rate and blood pressure. This can also cause severe angina. To ensure this doesn’t happen to our patients, taper, Taper, TAPER beta blockers!
Additionally, because they block the effects of norepinephrine, beta blockers can mask certain symptoms of hypoglycemia, including rapid heartbeat and tremor. The sign of hypoglycemia that remains intact even throughout beta blockade is diaphoresis, so diabetic patients should be counseled to monitor themselves for this remaining symptom.
Beta blockers have been associated with a multitude of adverse effects, including the following:
Fatigue (although this often improves with continued, consistent use)
Constipation
Dizziness
Nausea
Sexual dysfunction
Insomnia and/or nightmares (especially with the more lipophilic medications)
Weight gain/edema
Lastly, and there’s been a lot of debate and hullaballoo on this one… It may be prudent (if you have the flexibility) to be cautious when using non-selective beta blockers in patients with asthma or COPD. The thought here is that the non-selective blockade of beta-2 receptors may interfere with smooth muscle relaxation in the bronchioles, thereby exacerbating restrictive lung conditions. The other possibility is whether the beta blocker renders these patients’ beta agonist medications (rescue and maintenance meds!) ineffective by blocking their target sites of action in the lungs.
The most recent meta-analysis on this issue published in 2021 leans on continuing to use beta blockers when indicated due to the overall benefits - but perhaps being more cautious with propranolol specifically.
Clinical Pearls for Individual Beta Blocker Medications
Moving on to some particular pearls for individual agents…
Sometimes how it feels when you’re converting metoprolol between IV and PO. (Image)
We often think of beta blockers as relying heavily on the liver for metabolism and excretion. BUT, because there’s always an exception to the rule, atenolol requires a dosage reduction for renal dysfunction when the CrCl is less than 30 ml/min. So beware with the LOLs - the little old ladies who have been on this for years but are still taking the same dose they did 25 years ago.
Bisoprolol is one of the 3 preferred beta blockers for congestive heart failure (CHF), along with metoprolol (succinate) and carvedilol. (It’s not as frequently used though because it tends to be pricier than the other two.) Interestingly, it also has a renal dosing adjustment for CrCl <20 ml/min.
Esmolol and labetalol are both available IV and can be used in hypertensive emergencies. Labetalol’s alpha to beta blocking ratio varies based on route. When give orally, alpha:beta blockade is 1:3 in comparison to 1:7 when given IV!
Labetalol is also a safe antihypertensive option in pregnancy.
Metoprolol tartrate is the immediate release form of metoprolol. A good way to remember this is to think of tartrate as twice a day. Metoprolol tartrate is available IV, but this is usually used for urgent heart rate control rather than hypertensive crises due to its beta-1 selectivity. Notably, IV: PO is NOT 1:1. Rather, this ratio tends to be more like ~1:2.5 (ishhhh), although the dose should be closely monitored and titrated based on response after making the route conversion.
Metoprolol succinate is the extended release form of metoprolol. I like to remember this by knowing metoprolol succinate is a preferred beta blocker in heart failure and thinking “heart failure sucks” - like succinate, get it? When switching from metoprolol tartrate to succinate, the same total daily dose should be used.
Nebivolol is a special bird in the beta blocker family. Its claim to fame is that, in addition to being a selective beta-1 receptor antagonist, it also produces nitric oxide-dependent vasodilation. So it’s like an extra method for reducing systemic vascular resistance and lowering blood pressure. Before you get too nebivolol-happy, you should know it also has a renal dosing adjustment for CrCl <30 ml/min.
Propranolol… The beta blocker with undoubtably the most indications! Remember, it’s a “pro” for many “indications” (its brand name is Inderal). Because it’s highly lipophilic, it crosses the blood brain barrier and achieves high concentrations in the brain. This makes it useful for non-cardiac indications like migraine prophylaxis.
Sotalol is also an unusual bird in the beta blocker family. Even though it has beta-adrenergic blocking properties (obviously, that’s why it’s in this post!), it’s really mostly classified as a class III anti-arrhythmic and is used for atrial fibrillation. This is due to its predominant potassium channel blocking properties. Sotalol can cause QT interval prolongation, must be initiated in a facility that can monitor an EKG, and requires renal dosing adjustments for CrCl as high as 90 ml/min.
As a pharmacist, it’s important to know dosing, but honestly, you can always use your medication references to look up those details! On the other hand, knowing mechanisms, selectivity, and general place in therapy are useful starting points to ensure patients are maintained on the safest and most appropriate therapies. This is why we love pharmacology at tl;dr!!