NICU Series Part 1: Respiratory Issues in Premature Neonates
Steph’s Note: Today, I have the pleasure of introducing another new tl;dr writer to y’all. She’s not new to tl;dr, but she has (oh-so-happily!!) decided to expand beyond her current MPJE cheat sheet review role to share her pediatric-focused brain in written form. I’m so excited I’m gonna die!! (Kidding! But kudos if you get the Despicable Me reference.) So here’s to Courtney Howell, PharmD, and the start to her NICU knowledge drop series!
And BTW…we’ve got a super handy Pediatric Pharmacy Pocket Guide that gives you the ins and outs of all things peds. It’s an absolute life-saver…especially if you only occasionally come across pediatric patients in your practice. Check it out here!
Here’s to Agnes, the cutest steal-your-heart little cartoon girl ever. (Image)
Courtney graduated from the University of Kansas School of Pharmacy in 2020. After that, she stayed home with her kids for a year while her husband played Army (her words, not mine lol). They moved to Texas, where she worked as an inpatient pharmacist in a children's hospital. Late last year, they got an unexpected opportunity to move back to their home state and are loving being near family and friends again! She enjoys hiking, baking, a good rom-com, and the occasional (okay, more than occasional) overpriced Starbucks drink.
Picture this: It’s your first day rounding with the clinical team in the Neonatal Intensive Care Unit (NICU). A baby was just born prematurely at 27 weeks and 3 days (27w3d). The neonatologist starts rattling off alphabet soup. PMA, NRDS, NEC, ROP – what does it all mean?!
(Image)
The team decides to order Curosurf and asks Pharmacy for a dosing recommendation. What the heck is Curosurf? Isn’t that some type of seafood? Your preceptor turns towards you. You feel everything you’ve ever learned about neonates leave your body. In the distance, sirens.
Okay, it probably wouldn’t be quiiiiiite that dramatic, but starting a new rotation can feel overwhelming, especially when the subject matter wasn’t covered extensively in school. But never fear, tl;dr is here to help get you up to speed on all things neonatal intensive care!
This is the first installment of our NICU miniseries where we will break down potential complications of prematurity, management of infectious diseases, and nutrition in premature infants. Keep in mind we are only scratching the surface here. This series will mainly focus on complications of prematurity, but these and plenty of other complications can land a full-term baby in the NICU. We’re hoping this will at least serve as a solid foundation for you as you make your way into this new space. Today we will be reviewing potential complications of the respiratory system.
But first, the basics.
The NICU is a whole different world. Before we dive into the nitty gritty, let’s get oriented, shall we?
There are four levels of neonatal care defined by the American Academy of Pediatrics (AAP) ranging from basic care (level I) to subspecialty intensive care (levels III and IV). If you’re interested in learning more about the required capabilities and provider types, they can be found here in Table 1, but the main takeaway is that a higher number equals a higher level of care.
Two factors that play a part in determining what level of care a baby needs are age and size. Assuming no illness or complications, older, bigger babies generally require a lower level of care than younger, smaller babies. Birth weights are expressed as low (LBW: < 2,500 g), very low (VLBW: < 1,500 g), or extremely low (ELBW: < 1,000 g).
There are a few different terms you may see to describe age. The one we care about at birth is gestational age (GA). This is the time elapsed between the first day of the mother’s last menstrual period and delivery. The age at which a baby can survive outside of the uterus, also known as the age of viability, is clinically and ethically controversial. Depending on the source you look at, it ranges anywhere from 20 to 26 weeks GA. A baby is considered full term at 39 weeks GA.
After a baby is born, you may see a postnatal age (PNA), also known as chronological age. This is the time elapsed after birth. Postmenstrual age (PMA) encompasses everything since the first day of the mother’s last menstrual period, so it can be found by adding the GA and PNA.
Many babies need intensive care for weeks or even months after birth, so you may see a corrected age (CA) to account for prematurity. This can be found by subtracting the number of weeks a baby was premature from the PNA.
PMA is the preferred terminology during the perinatal period (around 22 weeks gestation to 1 week post-delivery), and the CA is the preferred terminology as a child ages. But they are essentially the same thing. A corrected age is used until a child is 2 years old. All of this terminology can get confusing, so let’s do a quick example to make it make sense:
First day of mother’s last menstrual period: 07/09/2022
Patient’s DOB: 02/20/2023
Today’s date: 04/18/2023
GA at birth: 32w2d
PNA: 8w1d
PMA: 40w3d
CA: 3d
(Image)
As you can see, this baby is just now considered to be full-term even though chronologically they are 8 weeks old. It may seem like these are just different ways to express age, but they can be important for correctly dosing medications.
For example, gentamicin is dosed primarily based on the PMA. This is because renal function isn’t fully developed until around 1 year of age, and PMA has been found to be strongly correlated with renal function and elimination of gentamicin.
You’ll notice a common theme as you work your way through this series. Essentially all complications arising from prematurity are the result of something being underdeveloped. And this makes sense, right? You wouldn’t take a cake out of the oven halfway through baking because it wouldn’t be done. Okay, maybe I shouldn’t liken babies to delicious cake, but the analogy holds up. I stand by it.
And now, back to the main event.
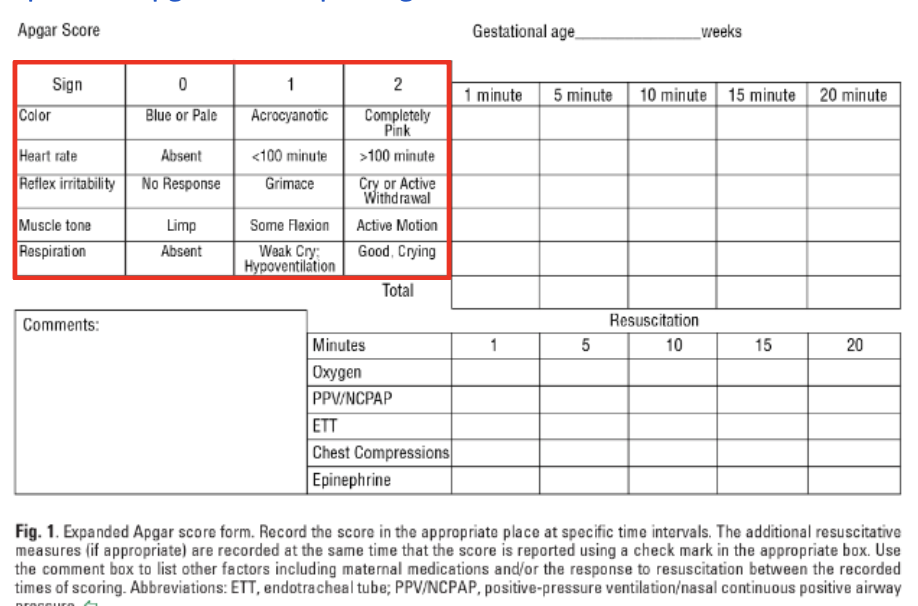
Whew. Now that we’ve gotten that out of the way, it’s time to get to the meat of this article. In case I haven’t given you enough new acronyms to learn, here is another one: Apgar. This is a scoring system used to assess an infant’s status after birth. It stands for appearance (color), pulse (heart rate), grimace (reflex irritability), activity (muscle tone), and respiration. The AAP and the American College of Obstetricians and Gynecologists (ACOG) encourage use of an expanded Apgar score reporting form that accounts for concurrent resuscitative interventions.
Scores range from 0 to 10. A score is assigned at 1 minute and at 5 minutes of life for all infants. A 5-minute score of 7-10 is defined as reassuring, 4-6 as moderately abnormal, and 0-3 as low. A score may be recorded every 5 minutes for up to 20 minutes for those that score less than 7 at the 5-minute mark.
Per the Neonatal Resuscitation Program (NRP) guidelines, the Apgar score should NOT be used to determine the need for initial resuscitation as resuscitative efforts must be initiated before the 1-minute score is assigned. It may be appropriate to discontinue resuscitative efforts if the Apgar score remains at 0 beyond 10 minutes of age. Infants with a low Apgar score may be suffering from respiratory complications.
Apnea of Prematurity (AOP)
AOP is one of the most common diagnoses in the NICU. The earlier a baby is born, the higher the risk of AOP. Because of this, infants born at less than 35 weeks gestation generally require cardiorespiratory monitoring after birth. The AAP defines an apneic spell as a cessation of breathing for 20 seconds or longer or a shorter pause accompanied by bradycardia (<100 beats per minute), cyanosis, or pallor.
Since bradycardia often accompanies apneic episodes, you may see these referred to as As and Bs. Many babies experience As and Bs during feeding, but these are generally not a problem as long as they resolve spontaneously and do not meet the criteria described above. Apneas can be classified as obstructive, central, or mixed.
Obstructive apneas are caused by an airway blockage, commonly pharyngeal collapse. These episodes can usually be managed with nonpharmacologic measures such as airway repositioning, oxygen, and CPAP.
Central apneas are the result of an immature brainstem and control of breathing. Oftentimes manual stimulation by means of a rub on the chest or a firm tap on the foot can be enough to pull the patient out of an apneic spell, but sometimes pharmacologic intervention is necessary. Methylxanthines have been the primary therapy for central AOP for decades.
NICU babies on caffeine, probably. (Image)
Both caffeine and theophylline are used in practice, but caffeine is preferred because it has a longer half-life and a wider therapeutic index. Therefore, it does not require therapeutic drug monitoring. If you’re into landmark clinical trials, the Caffeine for Apnea of Prematurity (CAP) Trial provided high-quality, reliable data on short-term and long-term outcomes of caffeine use for AOP. You can read all about the mechanism of action of methylxanthines in this AAP Clinical Report, but they essentially stimulate the central nervous system (CNS) and remind the baby to breathe.
Caffeine citrate is dosed as a 20 to 25 mg/kg IV or PO loading dose, followed 24 hours later by a 5 to 10 mg/kg IV or PO daily maintenance dose. Of note, the citrate salt is twice that of the caffeine base. To my knowledge, the only available liquid caffeine formulations are the citrate salt, so this probably won’t matter unless there is a crazy shortage and we need to start compounding our own. Regardless, it is a good piece of information to have in your back pocket.
Another pro tip: The IV solution can be administered orally, so you only have to stock one product and don’t need to re-make a dose if the route is changed from IV to PO. This would NOT work in reverse as a dose intended for PO use would not have been drawn up using aseptic technique. Let’s not create more problems for ourselves by introducing an infection risk, amirite? An oral solution is also available, so you may see both depending on what your institution stocks.
A trial off caffeine may be considered when a patient is no longer experiencing clinically significant As and Bs and has been off CPAP for 5 to 7 days or at 33 to 34 weeks PMA, whichever comes first.
AOP frequently occurs with gastroesophageal reflux (GER), but studies have shown GER is not likely to cause apneic episodes, and there is no evidence that pharmacologic treatment of GER decreases the risk of apneas in premature infants. In fact, gastric acid suppression in preterm infants may have harmful effects such as necrotizing enterocolitis (NEC - stay tuned, we will cover this later in the series!), late-onset sepsis (LOS - also coming soon!), and death.
Blood transfusions to correct anemia increase oxygen carrying capacity and respiratory drive, thereby reducing the frequency of apneas in the short term. However, there are no data to support long-term apnea reduction by means of blood transfusion.
An As and Bs-free period of time is required before a baby can be discharged from the NICU. This period of time is not well-defined, but the general consensus is that observation for 5-7 days is sufficient. The countdown usually begins a few days after caffeine is stopped to account for its long half-life (50-100 hours), and it only includes spontaneous As and Bs unrelated to feeding.
Neonatal Respiratory Distress Syndrome (NRDS)
Although many risk factors exist, respiratory distress and respiratory failure in premature neonates is primarily caused by pulmonary surfactant deficiency. If you dig waaaaaayyyyyy back into the pharmacology files of your brain, you’ll recall that surfactant is a substance made of fats and proteins that keeps the tiny air sacs (alveoli) in the lungs from sticking together.
Does anyone else’s brain try to pronounce alveoli like ravioli instead of al-vee-uh-lie? No? Just mine? (Image)
Production of surfactant begins around 24 weeks gestation but is not produced in sufficient amounts to prevent lung collapse until about 32 weeks. Consequently, babies born before 32 weeks gestation have a high chance of developing NRDS. Luckily, we have methods for both prevention and treatment of this respiratory complication.
An important tool in our arsenal for NRDS prevention is antenatal corticosteroid administration. Corticosteroids given to the mother prior to delivery can help speed up the maturation of fetal tissues and reduce the risk of several complications, including NRDS. ACOG recommends a single course of corticosteroids (betamethasone 12 mg IM Q24H x 2 doses or dexamethasone 6 mg IM Q12H x 4 doses) for pregnant women between 24 weeks and 33w6d gestation who are at risk for preterm delivery within 7 days. It may be considered between 22 weeks and 23w6d if neonatal resuscitation and support is planned. Antenatal corticosteroids are not currently recommended at less than 22 weeks gestation due to lack of beneficial data. Partial courses have also shown benefit, so corticosteroids should be initiated even if it is unlikely that all doses will be given.
Remember our poor pharmacy student who was tasked with dosing Curosurf? Well, they looked it up and found that it is indeed NOT a type of seafood (although that would be delicious). In fact, it is one of the available prevention (and treatment!) options for NRDS. There are currently three animal-derived (Survanta, Infasurf, and Curosurf) and one synthetic (Surfaxin) surfactants available in the U.S. Recommended dosing can be found in Table 2. Of note, Exosurf is no longer marketed in the U.S. due to lack of efficacy compared to animal-derived products.
Both prophylactic (prevention) and rescue (treatment) therapy have been shown to be beneficial; however, routine use of continuous positive airway pressure (CPAP) is considered superior to prophylactic surfactant therapy. Surfactant is typically administered via endotracheal tube, but several other methods are being practiced in an attempt to minimize invasiveness.
Bronchopulmonary Dysplasia (BPD)
Unfortunately, the solution to one problem can sometimes lead to another. BPD (also known as chronic lung disease (CLD)) is a long-term lung complication resulting from prolonged mechanical ventilation and supplemental oxygen. The term “BPD” has been used for decades and was based on specific pathological findings, so current terminology is moving towards CLD. I will use them interchangeably since most sources still refer to it as BPD.
The National Institute of Child Health and Human Development (NICHD) defines BPD as lung injury in preterm infants requiring ≥28 days of supplemental oxygen. Ventilation measures are usually initiated in infants with NRDS, so the prevention strategies for NRDS outlined above can also help prevent BPD. Our good friend caffeine also significantly lowers the risk of BPD, especially when initiated within the first three days of life. Its role in preventing AOP reduces the need for intubation, and it has been shown to facilitate extubation for ventilated patients.
We want to minimize the stabby stabbies as much as possible because even though they won’t remember it, neonates do feel pain!
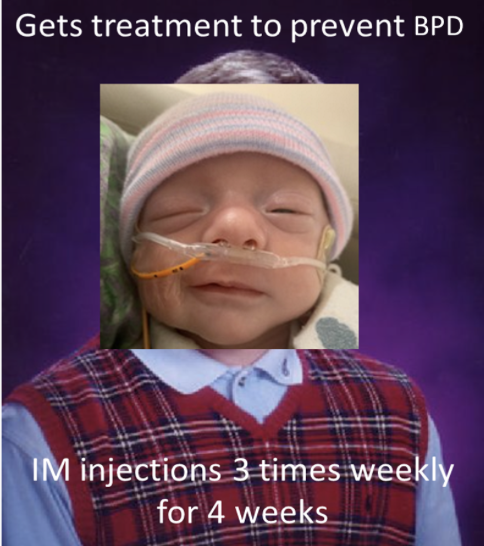
Vitamin A has also demonstrated benefit in preventing CLD. Vitamin A is required for the growth and differentiation of many cells, including those in the lungs. A large randomized controlled trial (RCT) conducted in 1999 found a slightly decreased risk of CLD at 36 weeks PMA in ELBW infants that received 5,000 units IM three times weekly x 4 weeks. As you can imagine, many clinicians are reluctant to subject their patients to painful injections three times a week. More research is needed to determine if oral administration of vitamin A would produce the same benefits.
Dexamethasone and hydrocortisone have been used for both prevention and treatment of CLD; however, routine use of postnatal corticosteroids (PCS) is not endorsed by the AAP as the risk of adverse events generally outweighs the benefits. High-dose PCSs (dexamethasone ≥0.5 mg/kg per day) are not recommended for treatment or prevention of CLD in preterm infants. If the team decides a PCS is necessary, a low dose for a short, predefined duration is recommended.
For example, the dosing protocol from DART (Dexamethasone: A Randomized Controlled Trial) is widely utilized to facilitate extubation. It consists of a cumulative dexamethasone dose of 0.89 mg/kg divided over 10 days.
Treatment with PCS should be discontinued if the infant does not show a clinical response within 72 hours. Inhaled corticosteroids (ICS) have been studied in an attempt to minimize systemic side effects, but no differences in effectiveness or adverse events were found.
Diuretics (primarily furosemide and chlorothiazide) have been used to reduce pulmonary edema and increase lung compliance. These are generally only used as needed due to the need for electrolyte monitoring and significant adverse events. This is especially true for furosemide as the half-life is prolonged in premature infants which increases the risk for ototoxicity. Data have not shown improvement in long-term outcomes of BPD.
Another class of medications used to increase lung compliance is bronchodilators. Albuterol is the most common, and use of a metered-dose inhaler (MDI) is preferred over nebulization because this method of delivery carries a lower risk of paradoxical increase in airway resistance. These are also primarily for acute symptom relief as data have not demonstrated improvement in long-term outcomes.
The tl;dr of Respiratory Issues in Premature Neonates
The biggest factor in a baby’s healthy development is time. Some babies are ready to party and arrive much earlier than others, and unfortunately this means their organs aren’t going to work the way they should. Luckily, we have a plethora of strategies to prevent and treat respiratory complications in premature infants.
AOP? Get that baby a coffee (jk, you should probably stick with caffeine citrate), oxygen, and CPAP.
NRDS? Prevent with antenatal corticosteroids for certain mothers at risk of preterm delivery within 7 days. Treat with surfactant and more CPAP.
BPD? Minimize invasive ventilation and supplemental oxygen as much as possible. Consider caffeine, vitamin A, corticosteroids (reserve for exceptional circumstances), and symptomatic relief with diuretics and bronchodilators. Researchers are working hard to find more options to better serve these patients.
And there you have it! Join us next time for a riveting discussion about patent ductus arteriosus (PDA)!