An Immune System Primer
Steph’s Note: I don’t know about y’all, but I feel like every other question I’ve been getting in clinic for the past year has been somehow related to the immune system.
Can’t imagine why. It’s been very…
How long am I going to be susceptible to COVID after getting my ocrelizumab infusion? Can I go back to work after my infusion?
Should we delay the second cycle of cladribine due to the risk of infection?
This patient who is already on an immunosuppressant needs to start Mavenclad, so when should he receive the varicella vaccine? Should he receive the varicella vaccine??
Seriously. Am I in a neurology clinic or immunology? Sometimes I wonder.
In light of these questions, it seems like now would be an opportune time for all of us to review at least the basics of the immune system. (Not that we’re going to answer all of the above questions, sorry. Each of them is a post in and of themselves.) Instead, we are going to investigate the background info working into our team decisions.
Let’s tackle this in a semi-organized manner…
Warning: the contents of this post are going to get personified. It’s simply too good of an opportunity to pass up.
What is the Immune System?
(Image)
Basically, street pharmacist Steph thinks of the immune system as your posse. This system has your back. It protects you from threats.
Normal pharmacist Steph would define the immune system as the culmination of cells, substances, and interplay of both of those that protect the human body from invasion or aberrance. That bacteria that tries to set up shop in your lungs…BAM. The immune system retaliates to keep you healthy (-ier).
(Generally speaking, of course…)
There are a couple of basic components of a well-functioning immune system:
It should be able to recognize what is a threat vs what is part of its own gang. The term for this is “self-tolerance.” Furthermore, it has to not only recognize the easily apparent threats, but it also has to be fiercely discerning to pick out imposters, undercover agents, and spies.
It has to be strategically located where it can attack threats. Or it has to be able to get there. Or if certain generals can’t get there themselves, they need to at least be able to send mercenaries to take out the threats. Basically, it has to be able to get from home base to the necessary site of war.
The different soldiers need to communicate effectively to coordinate battle.
It needs to have appropriate weaponry. Don’t bring a knife to a gunfight, right?
Innate vs Adaptive Immunity
We usually think of the immune system as having 2 branches. So instead of the military consisting of the Navy, the Army, the Marines, etc., the immune system has a military consisting of the innate and adaptive branches.
Innate immunity is like the infantry. It is the brute force that reacts quickly when a threat is detected, but these cells aren’t necessarily the most trained or strategic. In addition to cellular soldiers, innate immunity also encompasses things like barricades and environmental warfare.
So again, it’s not highly particular about targets but just sort of there to provide some additional hurdles for invaders to overcome.
Even though the innate immune system isn’t the most highly trained, the innate immune system is reeeeeally good at recruiting numbers to the site of action. Recruitment occurs via substances called cytokines and chemokines. Some examples of these include tumor necrosis factor (TNF) and interleukins (IL). Like the high schoolers’ brawl in the hallway that blows up super fast, the innate immune system yells out to get more fighters - quickly.
However, unlike the high schoolers’ fight in the hallway, the innate immune system is also really good at cleaning up the fight site. Innate immune system cleanup occurs by phagocytosis, literally meaning cells “eat” invaders and/or debris. In the chart below, you can see that both neutrophils and macrophages take part in phagocytosis, but others like eosinophils and dendritic cells may also phagocytose as well.
So metaphors and similes aside, let’s break these pieces down into actual cells and processes for a moment to bring it back to reality.
The innate cell soldiers are summarized in this table:
(Image)
Physical barriers of innate immunity include skin or mucous membranes. How about those cilia that sweep back mucous and all that it contains?
Environmental warfare can include changes in temperature or pH or the secretion of special chemicals.
It’s a crazy world in our bodies, isn’t it?! Total sci-fi alien warfare going on in there!!
Ok, let’s get back to analogies for a second to contrast innate immunity with adaptive immunity…
The Seals are legit. Rigorous training, equipped for the job, and ready for special missions. Very similar to our adaptive immunity forces. (Image)
Adaptive immunity is the Navy Seal force. These processes and cells are highly trained, picky about their targets, and remember former battles. Adaptive immunity isn’t usually the first set of fighters on the scene, but when they get to the action after a brief training delay, they’re there to do a job - and do it well.
The “memory” associated with adaptive immunity is key for developing future protection, as with vaccinations. The cells involved in adaptive immunity are the T cells and B cells.
What are T lymphocytes?
The primary lymphoid organs and some (but not all) of the secondary lymphoid organs. They’re EVERYWHERE. (Image)
T cells or T lymphocytes are one branch of the adaptive immune system. They are derived from multipotent progenitor cells called early thymic progenitors (ETPs). They are named as such because these undifferentiated cells must migrate from the bone marrow to the thymus organ to mature into their adult mercenary selves. (The bone marrow and thymus are also known as the primary lymphoid organs.)
For full nerd alert, here’s an analogy for you… ETPs : thymus :: Luke Skywalker : Dagobah. (Take that, high school SAT analogies!)
And huh, there is actually a purpose for the oft-undercelebrated thymus, and no, contrary to what I used to think, it’s not the same as the thyroid.
Anyways, within the thymus, the ETPs begin a pretty extensive maturation process, including differentiation into cells that express CD4 or CD8 (more on this in a few). They also acquire a T cell receptor (TCR) that is specific for a particular foreign body, aka an antigen. The maturing T cells then actually undergo positive and negative selective testing at several checkpoints to ensure they have been trained appropriately, will be functional, and won’t be autoreactive against the native body.
Immune system training is grueling, but necessary. I’m sure Yoda would agree. (Image)
It’s like the thymus has its very own Yoda testing the T cell trainees and making sure they won’t be traitors! Incredible.
So when T cells finally leave the thymus, they are known as naive T cells but only because these ruthless mercenaries haven’t been activated - not because they’re fresh-faced newbies who haven’t been through any crap. They’ve been debriefed on the enemy and put through rigorous testing to ensure they’re ready to fight.
What makes T cells effective?
T cells are responsible for the cell-mediated immune response. T cell activation and battle requires 3 components:
T cell recognition of an antigen via the TCR -> the T cell must be in the presence of its mortal enemy/target
Communication via co-stimulatory molecules -> the message has to be fully fleshed out and confirmed by other molecules
Clonal expansion -> the army has to grow in numbers to overpower the enemy and win the fight
Let’s look a little at each of these pieces…
For the first part about recognition of the enemy, the T cell needs to be in the right place at the right time for the TCR to bind to its specific antigen. If all the T cells are in the thymus after training, that’s not going to be super helpful, is it? That’d be like all the troops remaining at Fort Bragg after getting through boot camp. T cells need to be deployed to go on patrol, where they can interrogate invaders brought to them by antigen-presenting cells (APCs).
Location optimization is accomplished by T cell migration to secondary lymphoid organs in the body, which include the lymph nodes, the spleen, and mucosal-associated lymphoid tissues (MALT). FYI, there are various MALTs, including the GALT (gastrointestinal associated lymphoid tissues) and BALT (bronchial associated lymphoid tissues), as well as in the nasopharynx (e.g., tonsils), thyroid, breast, salivary glands, skin, and the eye.
Basically, there are LOTS of military bases to harbor the T cells as they patrol and interact with enemies at their most common entry points - the mucosal membranes! But T cells don’t just stay at home base either. They also go out on patrol in the bloodstream to increase their chances of finding and squashing enemies.
Then there’s the requirement that the TCR must be in the presence of its mortal enemy to activate the T cells. But luckily they’re not working alone here. T cells have a network of spies…
Several of the innate immune cells, including macrophages and dendritic cells, serve as antigen-presenting cells (APCs) for T cells. Other APCs include fibroblasts, epithelial cells, and even B cells (more later). But what you should take away for now is that these are the cells that are responsible for binding antigens and then tattling on them to the rest of the immune system. They do this by literally waving a recognizable antigen flag from their major histocompatibility complexes (MHCs).
Note how the MHC I is presenting parts of endogenous antigens within an infected cell to the TCR, leading to activation of CD8 T cells. In contrast, MHC II is presenting exogenous antigens from outside the APC to the TCR, leading to activation of CD4 T helper cells. (Image)
MHCs are congregates of proteins on the surfaces of APCs that are designed to hold pieces of a subdued antigen to flag down assistance from other parts of the immune system. (Call it brutal… but it’s not that different than sending an enemy’s body part in a box to signify the fight is on and to call for backup.) There are 2 types of MHCs:
MHC I, aka Human Leukocyte Antigen (HLA) A, B, and C: found on all nucleated cells, and
MHC II, aka HLA DP, DQ, and DR: only found on certain cells, including macrophages, dendritic cells, and B cells.
MHC I presents endogenous antigen fragments (usually peptides) to T cells, and when bound to the correct TCR, leads to activation of cytotoxic CD8 T cells. (Remember that every nucleated cell has MHC I, so that means every nucleated cell has the ability to throw up a flag for help!)
MHC II presents exogenous antigen fragments to T cells, and when bound to the correct TCR, leads to activation of CD4 T helper cells.
More on the subsets of T cells in a minute, but first, we need to finish talking about the activation process.
As far as the co-stimulatory molecules, these are other surface entities that can’t necessarily activate T cells by themselves. But like the first 9th graders on site who don’t want to join the action of a hallway brawl but have great lung capacity for yelling, they can confirm that a fight is going on and get more people at the site! So these co-stimulatory molecules can amplify the signals being sent out by activated T cells, which leads to increased production of interleukin-2 (another signaling molecule), increased T cell lifespan (so they can fight longer), and increase proliferation and differentiation of more T cells.
This leads us to the third piece, clonal expansion.
Not only are the originally activated T cells sending out signals that trigger additional T cell differentiation and proliferation, but the co-stimulatory molecules are also doing this. This leads to an onslaught of T cells coming to the site with an identical TCR as the originally activated T cell. The original brawler gets help from its posse, who is also trained to target the same mortal enemy.
Unlike high school brawls, after these immune system skirmishes, there’s a serious death phase in which ~90% of the activated T cells undergo apoptosis. This is called the T cell contraction phase. Not exactly the most appreciative welcome home for these immune system wartime heroes (and that name contraction phase would be such a coverup conspiracy if we were talking about our actual military!), but such is the way of the crazy sci-fi alien world of our bodies. The remaining small percentage of T cells that don’t apoptose during this contraction phase hang around to be sentinels for that same enemy if it shows up again.
Now that we have some info down pat about T cells in general, let’s take a closer look at different subtypes.
What are CD4 vs CD8 T cells?
Part of the T cell maturation phase in the thymus (aka while the T cells are training on Dagobah with Luke) is the process of differentiation into either CD4+ or CD8+ cells. Mature T cells can not be doubly positive for both of these markers. So what exactly are CD4 and CD8?
These stand for cluster of differentiation (CD) 4 and 8. These names are given to glycoprotein surface molecules that live on the surface of T cells and help to facilitate antigen recognition by the TCR.
CD4 T cells differentiate into T helper cells. These are named as such because they are the support crew for the mercenaries of the immune system, secreting cytokines to assist with signaling and jumpstarting B cell production of antibodies (more later). They have no killing or phagocytosis abilities. There are multiple subsets of CD4 T helper cells. Check them out in the figure below to get an idea of the cytokines they produce and their end helper functions:
(Image)
A small percentage of CD4 T cells differentiate into regulatory T cells, or Tregs. As their name suggests, they’re the referees of the immune system. They work to suppress escaped autoreactive immune cells that have the potential to cause autoimmune disorders or allergies. They do this by acting directly on T cell processes or indirectly by affecting APC processes. Unfortunately, because of their regulatory effects on T cells, they can also suppress normal anti-tumor responses, allowing for tumor growth. (Hey, nothing’s perfect…)
So then how are these CD4 cells different from CD8 cells?
CD8 T cells are also known as cytotoxic T cells. As you can probably guess, these are the actual mercenaries of the immune system military. They are cytotoxic, meaning they directly act on enemy target cells and kill them. Once contact is made between the cytotoxic T cell and the enemy, the CD8 cells commence some pretty ruthless, bone-chilling warfare:
Release of grenades of cytolytic granules, which contain a cocktail of pore-forming proteins (to punch holes and allow water entry, offsetting osmotic equilibrium, and also help with forced entry into the enemy cell) and proteases (to destroy enemy cell proteins, mitochondria, and DNA). These granule grenades also contain inhibitors of perforins to help protect the native T cell from the enemy cell’s own cytolytic defenses.
So not only are the cytotoxic T cells on the offensive with this assault, but they’re also on the defensive to prevent damage by the enemy. Ridiculously cool, right?
Expression of FasL (the Fas ligand), which attaches to the Fas receptor on the surface of the enemy cell. The Fas receptor is also known as the death receptor. (I’m not even kidding, that’s what it’s called. I didn’t make that up.) Through a series of chain reactions, the interaction of Fas and the FasL leads to enemy cell apoptosis.
In case you’re curious about this “death pathway”… DISC = death-inducing signaling complex. (Image)
So there you have it. An overview of where the T lymphocytes come from, how they differentiate into CD4 or CD8 cells and are trained to be a part of the adaptive immune system, and how they carry out their respective missions. Cold-blooded! (But not really - they’re doing it to protect us. I suppose we should just be glad they’re a) (usually) on our side and b) not external and like 10 feet in diameter. Now THAT would be scary.)
Now on to the other half of the adaptive immune system: the B cells.
What are B lymphocytes?
B cells or B lymphocytes are the other branch of the adaptive immune system. B lymphocytes are responsible for the body’s humoral, or antibody-mediated, immune response.
Much like T cells, B cells start out as multipotent stem cells in the bone marrow. However, unlike T cells, they develop into early lymphoid progenitors (ELPs), which is reflective of the fact that much of B cell maturation occurs within the bone marrow. (ELPs do not need to migrate to the thymus for training.) Part of the maturation process within the bone marrow includes the expression of a target-specific B cell receptor (BCR), or antigen-binding receptor, on the surface of each B cell.
Once the wee little B cells have matured sufficiently to be classified as immature B cells with their BCRs, they travel to the secondary lymphoid organs to become transitional B cells. Through a series of molecular signaling, they then become either marginal zone B cells or follicular B cells. Through a subsequent series of events, the follicular B cells then become the B cells we’re a little more used to hearing about: plasma cells or memory B cells.
See how the bone marrow is home to B cell development from hematopoietic stem cell (HSC) all the way through immature B cell. Also note the the immature B cell already has it’s Y-shaped surface antibody receptor attached at the time it leaves the bone marrow to go to the secondary lymphoid organs. So even though it’s not fully mature, it’s been trained enough to know what to target. (Image)
So what is this crucial series of events that gives us our final army of effective B cell subtypes…
What makes B cells effective?
The answer to this goes back to the BCRs. BCRs allow B cells to recognize antigens on their own without the help of APCs, unlike T cells which rely on other cells (APCs) to bring the enemy to their attention. When a BCR encounters the enemy it was designed to recognize, and depending on the cytokines and T helper cells present in the surrounding environment, the follicular B cells are triggered to differentiate into either an antibody-secreting plasma cell or a memory B cell.
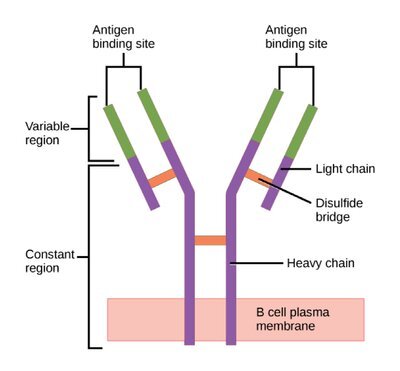
Structure of an antibody. Notice the 2 light (short) chains and the 2 heavy (long) chains that make up the backbone of the structure. Then there are the variable regions at the tips (green) that make each antibody a unique snowflake, capable of binding its specific target antigen. (Image)
Plasma B cells are fairly short-lived entities that really only exist long enough to eliminate the threat at hand. They do this by producing and secreting mobile versions of their same target-specific BCRs, and these heat-seeking missiles are known as antibodies. On the other hand, memory B cells stick around much longer and (as you might guess) provide the ability to recognize a past pathogen if it pops up again in the future.
So to clarify, antibodies are target-specific proteins that are expressed on the surface of B cells and can also be produced and secreted by plasma B cells. Longer-lived memory B cells express antigen-binding receptors (aka surface-bound antibodies) to provide continued surveillance for previously encountered enemies, and they can quickly be called into service to produce neutralizing antibodies.
There are 5 major subclasses of antibodies that are produced by B cells: IgM, IgG, IgD, IgA, and IgE. Each class has a specific function for recognizing and neutralizing certain categories of antigens, and within each subclass, there are numerous individual targets.
An important point here is that antibodies are not just the sentinels that sound the alarm when they recognize the enemy. You can also think of them as actively detaining and restraining the enemy until the killers can get to the scene. This is because antibody-antigen complexes serve as the catalysts or flags for several elimination methods, including the complement system, phagocytosing cells, and cytotoxic T cells.
(Image)
Check out the chart here to see the general functions of each antibody subclass.
FYI, like the regulatory T cell subtype (Tregs), there are also regulatory B cells (Bregs) that serve a similar purpose to keep the immune system in check.
It’s important to note that we’ve delineated out the T and B cells in a fairly distinct fashion to help keep them straight. However, these 2 branches of the immune military are absolutely not mutually exclusive! B cells can serve directly as APCs for T cell activation, and their antibody products often flag the enemy for T cells to destroy. On the other hand, T cells often serve in helper roles to facilitate the differentiation and proliferation of B cells. So just to be clear, these processes are interdependent.
Additionally, the innate and adaptive branches are also not mutually exclusive! In addition to B cells receiving assistance from T helper cells, B and T cell stimulation and proliferation can also be influenced by various parts of the innate immune system, including natural killer cells, dendritic cells, macrophages, neutrophils, basophils, and eosinophils, to name a few.
What are CD19 and CD20 B cells?
CD19, or cluster of differentiation 19, is a B cell surface co-receptor for the main BCR. It is present from the early stages of B cell maturation (pro-B cells) all the way through mature memory B and plasma B cells. Because of this wide array of B cell subtypes that express this protein, it is often used as a biomarker for B cells. (CD19 is present across even more subtypes than another better-recognized but alternative marker protein, CD20!)
See how CD19 is basically the most prevalent and enduring surface protein expressed through B cell development? This is what makes it a good B cell biomarker! (Image)
So unlike the use of CD4 and CD8 to differentiate different types of T cells, the CD19 and CD20 biomarkers don’t necessarily help us to differentiate between B cell types and functions - because they’re present on a large portion of B cells. But they do help us to count B cells in general… Plus, CD19 cell counts can be especially useful when the CD20 protein may not be available for quantification due to binding by anti-CD20 medications, like rituximab or ocrelizumab.
The tl;dr of the Immune System
The human immune system consists of a very complex network of cell types, signaling molecules, and methods for destruction of pathogens. No part is completely independent of the others, and when one part is dysfunctional, this can lead to issues like hypersensitivities, autoimmune disorders, and immunodeficiencies.
In my best attempt to summarize these complicated processes… The innate immune system consists of the cells, environmental, and physical barriers that initially encounter foreign entities. It’s not particularly well-trained to target specific enemies. But by golly, it’s fast, and it’ll throw its brute force around.
The adaptive immune system consists of more specialized and trained T and B cells that are activated by each other (as well as potentially parts of the innate immune system and various signaling molecules) to seek and destroy specific targets. It also holds memory so that past pathogens can be recognized again if needed in the future with antibodies playing a large role in this capability to remember.
I’m going to leave you with this pretty awesome summary figure to spend a little extra time with when you feel ready…
(Image)