A Pharmacist's Primer on Orphan Drugs
Steph’s Note: Hi everyone! Glad to be back after my sleep-deprived (but very snuggly) baby hiatus! This week, we’re taking a little break from the deep clinical dives to tickle the regulatory and administrative sides of your pharmacist brains. We’re all pretty familiar with the pharmacotherapy for the hard hitters - diabetes, hypertension, hyperlipidemia, etc. But what about some of those disease states that aren’t as common? How are those medications developed, and how is it financially viable for them to exist?
Who could resist this face?
To answer these questions, I’d like to introduce Philip Moliterno. He’s a recent 2023 graduate of St. John’s University. He worked at a big corporate pharmacy for the majority of his school life, and he also worked for a specialty pharmacy as a graduate intern, counseling patients extensively on rare diseases, REMS drugs, and oral chemotherapies. Now he works for a major hospital system in his area, doing quality management, addressing regulatory standards, and answering drug information questions. In his free time, he loves cooking, listening to pharmacy podcasts, and most of all, hanging out with his fiancé and their dog Luna, who likes to throw the ball at his laptop when she wants him to stop working! Teach us, Phil!
Let’s Talk about “Rare” Diseases
First, let’s talk about what I mean by a rare disease. I remember hearing the phrase, “When you hear hoofbeats, think of horses, not zebras.” And I thought to myself, “What the heck does that even mean?” Maybe you thought the same thing.
It means this - think of the most common causes of symptoms before you think of the unique causes. Usually, when you hear hoofbeats, it’s a safe bet to think horses. Basically, if it walks like a duck and quacks like a duck, it’s usually a duck.
That being said, today we’re talking about the zebras…the less suspected sources of those hoofbeats.
Analogies aside before this turns into a total zoo.
To define a disease as rare, fewer than 200,000 Americans must be living with it. Or, alternatively, it can be a disease that affects more than 200,000 people, BUT the developer cannot reasonably expect to recover the cost of developing and marketing a treatment.
Right… “Rare” diseases… (Image)
With over 10,000 rare diseases affecting more than 30 million patients, rare diseases are “rare.” I’m willing to bet you know quite a few of these “rare” diseases. Heard of amyotrophic lateral sclerosis (better known as ALS or Lou Gehrig’s disease)? Sickle cell anemia? Acute lymphoblastic leukemia or Huntington’s disease?
Yeah, not as uncommon as you might think.
So how do treatments for these not-so-rare (but still relatively rare) diseases exist?
Developing Treatments for Rare Diseases
Luckily, there are often drug therapies available for these rare diseases (yipee). Any medication used to treat a rare disease is considered an orphan drug. While these drugs might not be stocked at the local corner store, even if it is owned by a billion dollar corporation, they are available on the market.
Even Mr. Monopoly wants to know why these meds are so pricey! (Image)
Enter stage right, the specialty pharmacy. This type of pharmacy provides services for regimens that are complex, have special requirements, or are generally just considered not suited to be kept at a local pharmacy. Sounds like a pretty darn good fit for treating patients with rare diseases! On top of that, medications for rare diseases are expensive for patients (so expensive that Mr. Monopoly wouldn’t be able to pay out of pocket for it), and specialty pharmacies often have resources to help patients navigate medication access.
Why are these medications expensive?
I’m glad you asked. Drug companies pour money into researching potential drug targets, formulating and creating the drug, and then funding clinical trials to prove the safety and efficacy of the drug (i.e., get FDA approval). Selling their approved drug products is how these companies generate profit.
This last part is where it can get sticky for orphan drugs, for which the target patient population is miniscule compared to the patient populations of other disease states (think diabetes or hypertension). Basically, there are fewer people to sell the drug to in rare diseases, making it more difficult to generate a profit. This is why the FDA passed the Orphan Drug Act.
And now for the meat and potatoes of the post.
How the FDA Uses the Orphan Drug Act for Rare Diseases
The Orphan Drug Act defined what it means to be an orphan drug and why drug companies should consider developing them. The act also requires companies to submit annual updates regarding the status of drug therapy trials and drug formulation. This information can be submitted through mail, email, or the CDER NextGen portal, which is essentially the online communication link to the FDA.
CDER, aka the Center for Drug Evaluation and Research, is the branch of the FDA that regulates prescriptions and OTC drugs to make sure what’s available is both safe and effective. While it doesn’t conduct the studies, CDER will actually help manufacturers design study endpoints and hone necessary information for FDA approval, and it reviews all the study results submitted by manufacturers. CDER even supports an Accelerating Rare disease Cures (ARC) program, whose mission is to speed availability of viable treatments for rare diseases.
It really is pretty cool how many resources are out there to help develop new treatments!
Speaking of… The FDA also helps cover some costs of human testing in the form of orphan drug grants. Currently for 2023-2027, the grant provided is $30 million! There are further grants available to companies studying treatment for Lou Gehrig’s disease and other degenerative neuromuscular disorders. In addition to the grant, companies are allowed to claim a tax credit of 25% of their costs for clinical testing while developing a rare drug.
Drug companies during that period of market exclusivity. (Image)
In addition to available grant money, there are other incentives for manufacturers to work on orphan drugs. The manufacturer is guaranteed 7 years of market exclusivity after initial approval, meaning no generic versions can be marketed during that time. For pediatric rare diseases, priority review is provided by the FDA, meaning the FDA will approve (or not approve) a drug within 6 months of the application being submitted.
Some special notes about requesting pediatric orphan drugs designation… For manufacturers seeking a pediatric medication indication, the term rare disease is still defined as above. That is, it accounts for all patients, regardless of age. So although a certain disease may be rare in children, it may not be rare in adults, and therefore would not qualify for orphan drug designation!
Another unique caveat is that the company cannot be seeking orphan drug designation for a medication that is used for a different adult indication. (That would be like double dipping on the money end!) Pediatric orphan drugs also must have been studied in a pediatric population, rather than having data extrapolated from adult studies. (Kiddos are NOT just little adults when it comes to drugs, as we know from our NICU series!)
Now that we’ve covered some of the ways the FDA encourages and supports orphan drug development, let’s take a look at a real world case.
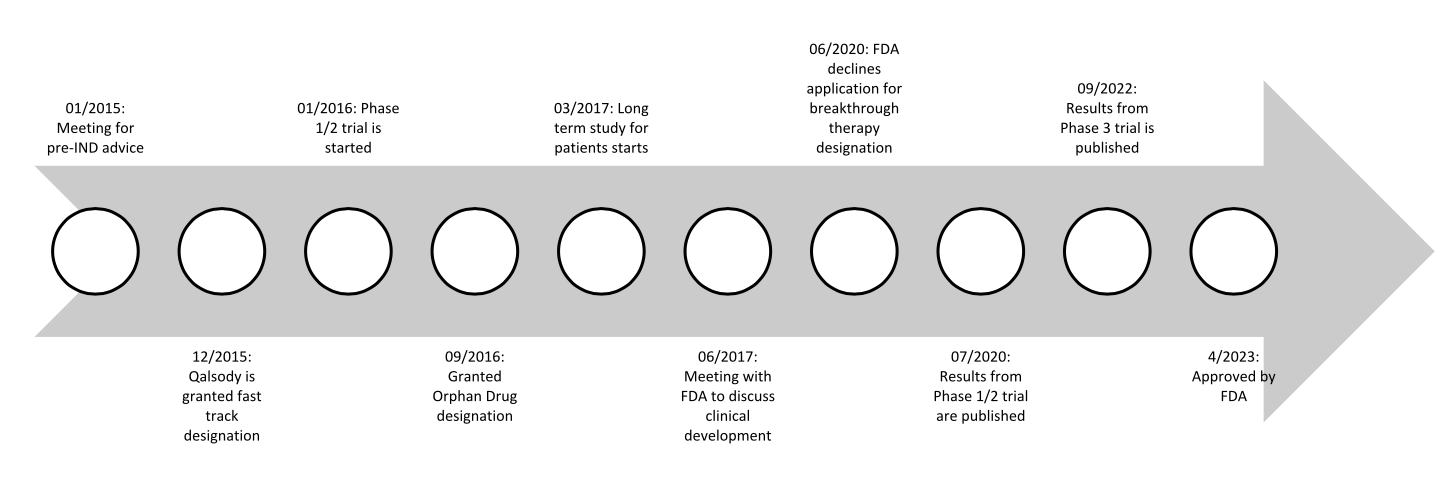
One drug recently approved is Qalsody (tofersen) sponsored by Biogen in collaboration with Ionis. This medication was granted orphan designation in September 2016 and then approved for the treatment of SOD1 mutated ALS recently in April 2023. Almost 7 years later! And there’s still an ongoing confirmatory trial. Qalsody is an intrathecal injection of an antisense oligonucleotide that reduces synthesis of the SOD1 mutated protein. The mutated protein prevents the breakdown of toxic metabolites from normal cell processes, leading to accumulation and the damage associated with ALS.
Let’s dive deeper into Qalsody’s development journey. Starting before its orphan drug designation, Biogen was able to meet with the FDA regarding pre-IND advice in January 2015. This type of meeting generally is not designed for the FDA to lay out explicitly what it will take for the proposed drug to be approved. Rather, this meeting allows for open communication between companies and the FDA. It allows the FDA to provide advice to the company for what they expect to see during development.
In the case of Qalsody, this pre-IND meeting was used to discuss what non-clinical studies of toxicology could be used to lead to the IND approval. In November of the same year, an IND was granted. In December, Qalsody was granted fast track designation.
A Phase 1/2 study was started in January 2016, when the drug was still designated as BIIB067. This Phase 1/ 2 trial addressed the pharmacokinetics, pharmacodynamics, and safety and tolerability. Results were published in July 2020.
This trial was expanded to a Phase 3 trial with the majority of the patients involved in the previous phases advancing to this next phase. Results for the Phase 3 portion were published in September 2022. A concurrent study for the long term effects for any patient who had completed any part of the previously mentioned studies was started in March 2017 and is expected to be completed June 2024.
While the clinical studies were ongoing, Biogen also had plenty of responsibilities on the business side. The company had a meeting with the FDA in June 2017 to obtain feedback for how to proceed with the clinical development of Qalsody. May 2020 was when Biogen met again with the FDA to discuss the “enrichment” of the primary study population. Although they suffered a minor setback in June 2020 when the FDA denied the application for breakthrough therapy designation, Biogen was able to meet again multiple times with the FDA to discuss statistical analysis procedures and results ahead of submitting the full NDA.
In April 2022, Biogen met with the FDA in a meeting specifically for discussing how the IND would be laid out. The NDA was submitted in May of the same year for approval through the accelerated approval pathway. In July 2022 priority review was requested, and it was granted in October 2022, with its ultimate approval in April 2023.
A drug 7 years in the making…! Here’s a summary of its orphan drug journey:
That’s just one example of an orphan drug! Other examples of treatments for rare diseases include…
It was designated as an orphan drug in December 2015 to treat sickle cell anemia. It is the first and only in-class hemoglobin S polymerization inhibitor.
Hemoglobin S is mutated hemoglobin that is produced in people living with sickle cell. It does not carry oxygen as efficiently and is primarily to blame for the symptoms and downstream effects of the disease.
Doxorubicin
It is used off-label to treat cancers that are also considered rare diseases, like acute lymphoblastic leukemia. It is unique due to its origin. It is isolated from a mutant strain of Streptomyces peucetius and is then treated with a chemical to create doxorubicin.
For more examples of recently approved orphan drugs, check out the ARC program’s website here!
The tl;dr of Orphan Drugs
As pharmacists or those working in a pharmacy, we are often a first line of communication for patients. Although most community pharmacists might not be filling orphan drugs, or inpatient pharmacists might not have orphan drugs filling up the Pyxis machines, we can still provide value to patients on a more personal level by providing resources to patients. We should at least be familiar with CDER and their ARC/Rare Diseases team so that we can direct patients and/or providers to their site for more information!
The NIH also sponsors GARD (the Genetic and Rare Disease Information Center), which provides a comprehensive search engine for rare diseases. Each disease state has a portion dedicated to patient organizations, which can provide useful and important information to patients. Just another example of an important resource for pharmacists to be familiar with!
To summarize, even though orphan drugs aren’t as common as our antihypertensives or antidiabetic agents, they certainly aren’t necessarily as rare as the designation implies! Orphan drugs are a force to be reckoned with, and understanding the regulatory aspect of their development is one step into a greater journey. Thank you for reading!