The ABCs of LVADs for Pharmacists
Steph’s Note: Y’all are in for a major treat with this one. But if you haven’t already, you probably want to review the our 3 part series on congestive heart failure. It may also help to read about warfarin and anticoagulation in general! Gotta have the basics before you can dive into the deep end, yay? But then when you do dive, well all I can say is that I legitimately learned more about LVADs in this one post than I think I did in all of pharmacy school AND residency! So who’s bringing the knowledge bacon here?
Dr. Ashley Barlow is a PGY1 Pharmacy Practice Resident at the University of Maryland Medical Center in Baltimore. She received her pharmacy degree from Jefferson College of Pharmacy in Philadelphia, Pennsylvania. Following her PGY1 year, Ashley is driven to pursue PGY2 training in either oncology, cardiology, or critical care. Ashley is passionate about academia and is driven to attain a job in an academic medical center to serve as a clinical pharmacist who engages in didactic teaching. Ashley is actively engaged in social media on her collaborative twitter account @theABofPharmaC with her sister Brooke Barlow, where they extend their creativity and passion for clinical pharmacy by designing innovative learning tools to promote the intellectual growth of their followers.
Dr. Brooke Barlow is a PGY1 Pharmacy Resident at the University of Kentucky Healthcare in Lexington. She completed her Doctor of Pharmacy at the Jefferson College of Pharmacy. Her clinical areas of interest include critical care, cardiology, and solid organ transplantation. She is passionate about engagement on social media platforms such as twitter, as it engages her commitment to learning and strive for continuous professional development. Outside of pharmacy, Brooke enjoys healthy cooking, weight lifting, and photography!
They are joined on this post endeavor by Craig Beavers, PharmD, FACC, FAHA, BCCP, BCPS-AQ Cardiology, CACP. Dr. Beavers currently serves as the Director of Cardiovascular Services at Baptist Health Paducah, Cardiovascular Clinical Pharmacist with University of Kentucky Healthcare, and Adjunct Assistant Professor with the University of Kentucky College of Pharmacy. He also serves as the Chair of the American College of Cardiology Cardiovascular Team.
Now THAT’s a POWERHOUSE team. Let’s get to learning!
And BTW…if you’d like a downloadable (and printer-friendly) version of this article, you can get one here.
Heart failure (HF) is a chronic, progressive condition that affects nearly 5.8 million adults in the U.S. and ~23 million world-wide. Despite the advent of guideline directed medical therapy (GDMT) and associated improved survival, it is estimated that ~10% of patients will progress to advanced heart failure (AHF), (stage D; refractory HF requiring specialized interventions). AHF is associated with an exceedingly high rate of morbidity and mortality, whereby the only definitive treatment is a heart transplant.
However, the number of potential candidates far exceeds the donor pool, excluding transplant as a potential life-saving intervention for many patients. Historically, management of AHF consisted of inotropic therapy, but these treatments were substantially limited by the development of life threatening arrhythmias, tachyphylaxis, and other serious toxicities. Due to the associated risks with use of inotropic therapies and the limited donor pool available for transplantation, the advent of left ventricular assist devices (LVADs) have revolutionized the landscape of AHF treatment. This intervention has demonstrated improvements in morbidity, mortality, and quality of life, dramatically shifting the prognosis of AHF.
Unfortunately, the significant survival benefit observed with LVADs is counterbalanced by the high rate of severe complications, occurring in up to ~70% of patients post implantation.
With an understanding of device mechanics, pharmacologic interventions, and potential complications, pharmacists can play a fundamental role in the multidisciplinary care team to optimize the pharmacological treatment and improve outcomes for patients with LVADs.
What is an LVAD?
One example of an LVAD system.
An LVAD is a durable, implanted, mechanical circulatory device that can either supplement or fully support the cardiac output (CO) for a poorly functioning heart. The device augments systemic perfusion by propelling blood in a forward fashion, resulting in a decrease in cardiac workload, increase in CO, and improvement in oxygenation to vital end organs.
LVADs, as the name suggests, provide support for only the left ventricle (LV). This is important because candidates best suited for an LVAD are patients with preserved right ventricular (RV) function with isolated LV dysfunction, a common consequence observed in left sided HF. The duration of LVAD implantation can vary from a few weeks to a lifetime depending upon the treatment trajectory.
Short term LVADs are employed as a means of hemodynamic support to bridge patients to a heart transplant, which may be a few months to years in duration. Short-term VADs may also be used as a form of dynamic support for cardiogenic shock refractory to medical management as a bridge to myocardial recovery.
Permanent LVADs on the other hand are implanted for life-long support is used as “destination therapy” for patients who are not candidates for transplant and/or who are refractory to or intolerant of inotropic support.
Speaking of LVAD candidates… See the INTERMACs info below:
Image adapted from here.
Types of VADs
The evolution in LVAD designs over the years has led to significant improvements in hemodynamic support while reducing the risk of potentially fatal complications. First-generation LVADs generated “pulsatile-flow”, meaning the delivery of blood flow from the left ventricle to the aorta was driven by mechanics that mimicked the heart’s physiologic “pumping” function.
Although first-generation devices demonstrated improved survival rates, they had several flaws that limited their routine use. Not only were they bulky, large, titanium based (yes...titanium..) devices, the device had several extracorporeal pieces that resulted in a high rate of infectious complications. Furthermore, the first-generation devices had limited durability, with only a 5 hour battery life, which required patients to be vigilant of the duration of their activities off of the charger to ensure the device had adequate power to support hemodynamic circulation.
Second and third-generation LVADs are referred to as “continuous flow” devices, which function to generate blood flow by a single rotary element that propels blood forward from the left ventricle through the aorta.
Pulsatile versus continuous LVADs. (Image)
Continuous flow devices are further categorized as “axial flow” vs “centrifugal flow” based on the rotary device that facilitates blood movement.
Think of axial pumps as blood running through a pipe, in contrast to centrifugal, which utilizes a spinning bladed disk to propel blood forward. In general, these later-generation devices are smaller, more durable, reduce the incidence of complications, and improve mortality compared to first-generation devices.
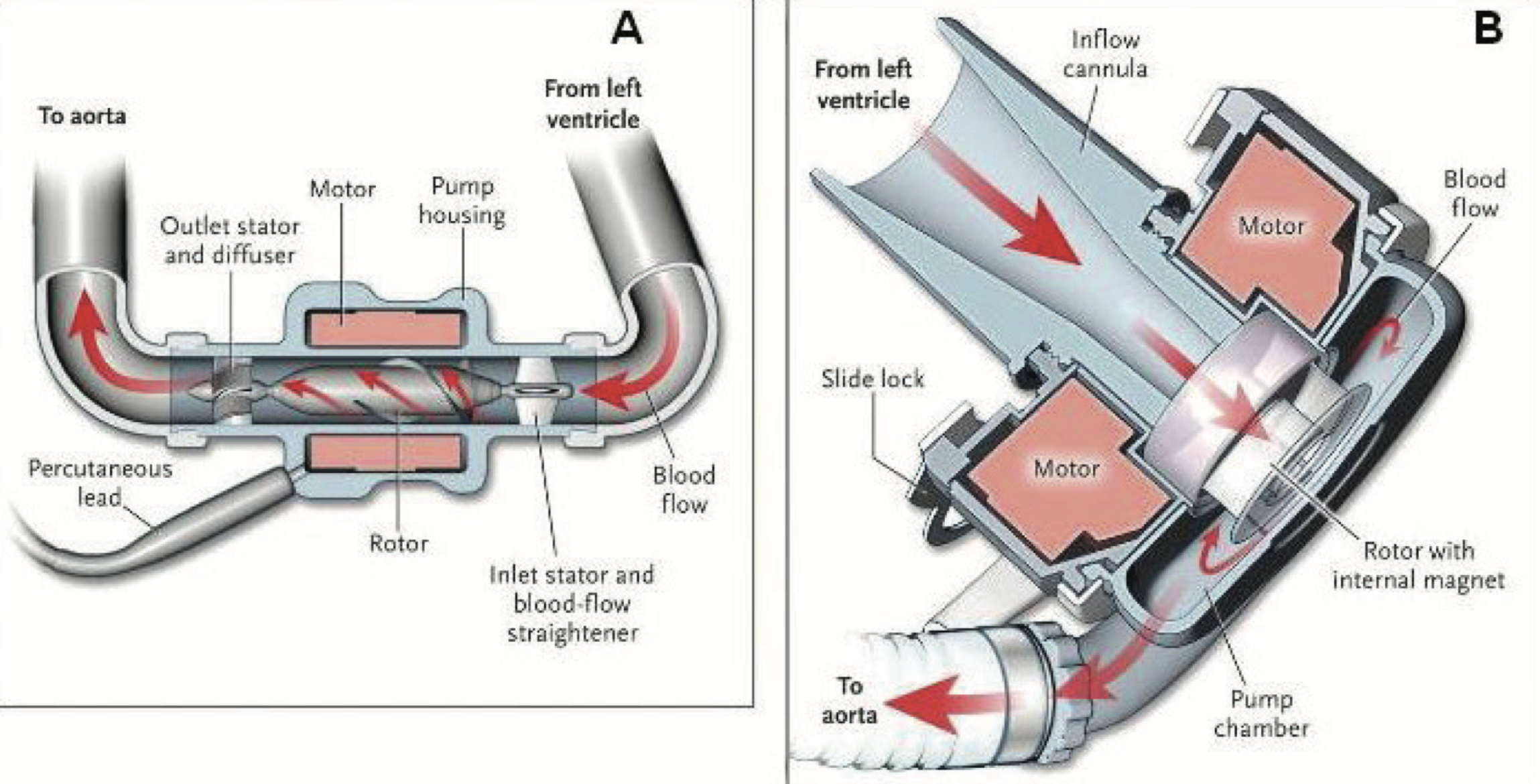
Panel A is an axial flow continuous LVAD. Panel B is a centrifugal-flow continuous LVAD. (Image)
Regardless of the generation of LVAD, each shares the same basic design and components.
Each device contains an inlet cannula that is placed in the apex of the left ventricle, a pump, and an outlet of the ascending aorta, which is placed in a tract that aligns with the movement of blood flow through the left ventricle. The most essential component to these devices is the battery pack.
Yes, patients have to physically plug themselves in to “recharge” their mechanical hearts, making this intervention limited to patients with reliable access to electricity.
LVAD Device Mechanics
There are four parameters that are essential for monitoring LVAD functioning; think the 4 P’s:
Pump Speed is a measure of how fast the rotor is spinning that determines the amount of blood flow through the left ventricle. As the pump speed increases, the rotor spins quicker and is capable of circulating a greater amount of blood volume. This will relieve pressure from volume overload on the LV and restore cardiac output. This is the only parameter that is programmed and controlled by the physician, and will not change based on patient hemodynamic status. As a matter of fact, the other device mechanics described below will all change in order to maintain the fixed pump speed.
Pump Flow is generated by the speed of the rotating impeller and depicts the amount of total circulating blood volume out of the left ventricle. Think of this value as a synonym to cardiac output which is measured in L/min. Normal values range 4-6 L/min.
Pump Power is the amount of energy required to spin the LVAD rotors at the specific speed that has been pre-set by the physician. When flow is indirectly obstructed (i.e., not directly in contact with the rotor) power decreases. Power increases when flow through the rotor is obstructed, such as when a thrombus forms on the rotor.
Pulsatility Index is the magnitude of change in blood flow when accounting for heart rate and contractility over a 15-second interval. This value depends on the amount of circulating blood volume available for forward flow as well as the contractility strength of the native heart. The pulsatility index is inversely related to the amount of assistance provided by the pump with unloading of the LV, i.e., the higher the pulsatility index the more the patient’s native heart is functioning. The pulsatility index decreases when there is low circulating blood volume or an obstruction associated with thrombosis.
A little background info for our example. (Image)
Let’s put these factors into an example to see how you could use these parameters to guide clinical decision making for pharmacological interventions.
Now the example.
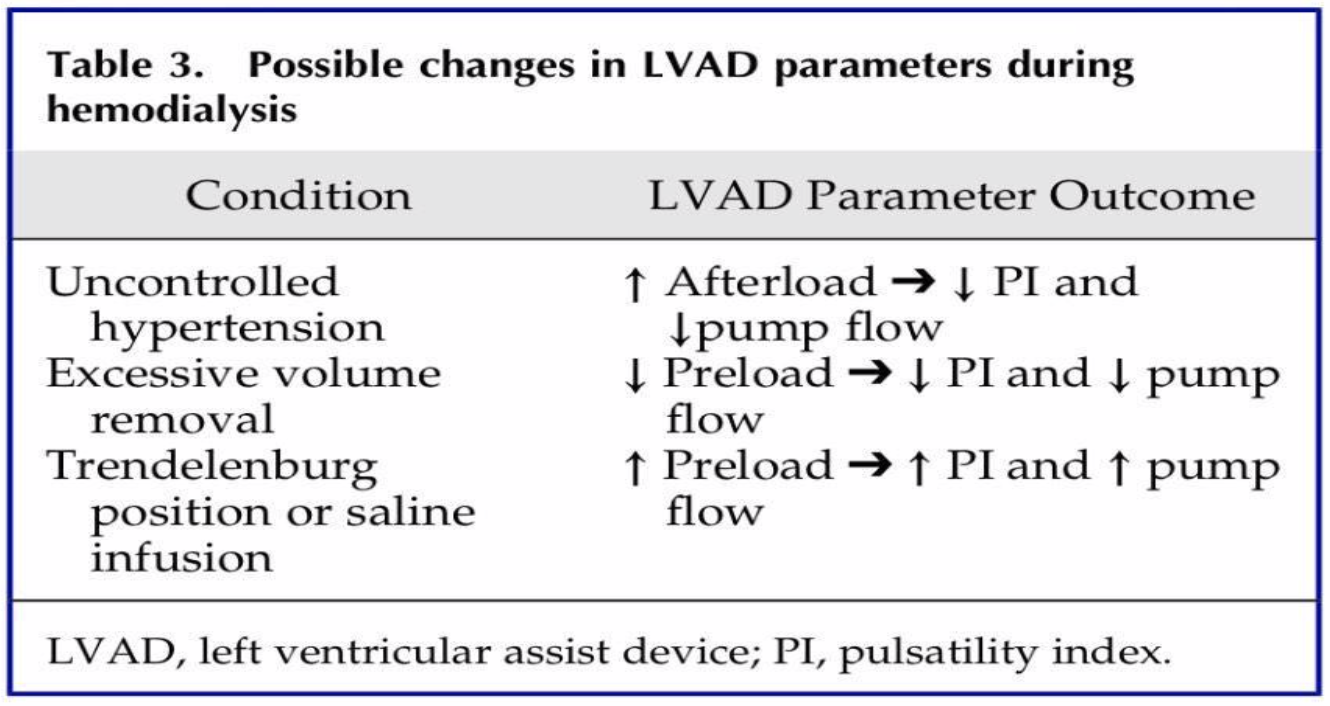
Let’s say an LVAD patient presents to your cardiac critical care unit with a low pulsatility index and a low pump flow. A little history, he has had heart failure for 15 years and received an LVAD 3 weeks ago as a bridge to transplant. While his dynamic variables are being adjusted, his outpatient physician initiated him on furosemide 80mg twice daily due to symptoms of volume overload and increasing dyspnea.
On admission, his BUN and serum creatinine are elevated, which is suggestive of dehydration. In this scenario, LV preload (total volume in the LV) is reduced, which would decrease cardiac outflow because of decreased total circulating blood volume. (This is the second row in the above chart -> excessive volume removal.)
The pulsatility index is low because the pump has to work harder to maintain the same cardiac output and flow since the patient’s volume and native heart function are low.
After understanding the patient’s LVAD parameters, we can now recommend small boluses of intravenous fluids to reverse overdiuresis and return the LVAD parameters back to baseline.
This situation is common and is also called a “suction event” because if the LV preload drops too low, the walls of the left ventricle could collapse enclosing the device within them.
Complications of LVADs and Their Management
So now that we have a background understanding of LVADs, we can now dive into the complications of LVADs. Complications of LVADs can be broken down into device-related, patient-related, or interaction between the patient and the device:
The management of LVADs requires a careful balancing act, as any acute changes can tip patients from one extreme to the next (bleeding or thrombosis, hypertension or hypotension). Pharmacologic prophylaxis remains a cornerstone therapy for patients with LVADs to prevent complications, specifically thrombosis, where we as pharmacists can play an essential role in optimizing the care for these patients.
LVADs and Blood Pressure
Continuous flow LVADs lack “pulsatility” compared to the pulsatile devices. The absence of a palpable pulse with these newer devices results in the inability to measure blood pressure by auscultation. Blood pressure is best estimated by a doppler ultrasound probe and sphygmomanometer to target a goal MAP of 70-90 mmHg. Blood pressure management is crucial for patients with LVADs to minimize the risk of fatal consequences such as aortic valve insufficiency, ischemic strokes, or intracranial bleeding.
Pharmacologic management should be centered around afterload reduction, which is the major driver for increased MAP. It is important to remember that LVADs do not “cure” HF; therefore, optimizing GDMT with beta blockers (carvedilol, metoprolol succinate, bisoprolol) and ACEi/ARBs is vital to achieve the mortality benefits associated with these therapies. ACEi/ARBs can effectively reduce afterload and blunt the sympathetic surge associated with RAAS (Renin Angiotensin Aldosterone System) activation, with the additive benefit of reducing the risk of arteriovenous malformations (AVM).
Non-dihydropyridine calcium channel blockers, such as verapamil or diltiazem, have negative chronotropic effects and should be avoided for blood pressure management due to the risk of precipitating right sided heart failure.
LVADs and Thrombosis
Thrombosis is one of the leading causes of hospitalization in patients with LVADs. Given the high prevalence of atrial fibrillation among patients with HF, ischemic strokes account for the largest percent of thrombotic events. Unique to patients with LVADs, hypertension and infection also play an important role in the pathogenesis of thrombotic events and have been identified as independent risk factors. Complicating matters is that thromboses can also arise secondary to device implantation.
Thinking back to our clotting cascade, turbulent blood flow through the device causes shear stress to platelets, which acts as a stimulus for tissue factor expression and subsequent activation of the coagulation cascade. This can be a devastating complication, as the obstructive clot within the device or its conduits can impair pump performance and diminish CV support.
Due to the life-threatening nature of thrombosis and the complex interplay between platelet and clotting factor activation, prophylactic anticoagulant and antiplatelet therapy is a critical component in the care of all patients with LVADs.
At their initial approval, LVAD manufacturers made specific recommendations for preventive antithrombotic treatments of varied intensity within their package insert that were distinct for each device. Regimens included a combination of warfarin, aspirin, +/- dipyridamole. However, due to the concomitant high risk of bleeding (discussed below), the need for triple therapy with high INR targets has been called into question.
The International Society of Heart and Lung Transplantation (ISHLT) guidelines have different anticoagulation targets based on real world data demonstrating similar efficacy and improved safety. As pharmacists play a critical role in monitoring and managing anticoagulation, it is crucial to know which guidelines your service follows to adequately assess and adjust warfarin doses.
A summary of thrombosis prevention in LVAD patients - and a little info about how often these combos are used. (Image)
In clinical practice, dipyridamole is rarely, if ever, employed, due to the higher tendency of bleeding, headache, and GI complications that can result with this agent. In patients with an allergy to aspirin, clopidogrel is an appropriate anti-platelet substitution. LMWH can be considered during periods of subtherapeutic INRs or for perioperative bridging, but it is not recommended as a long-term alternative to warfarin.
You may be thinking to yourself, what about the direct oral anticoagulants (DOACs)?
Apixaban has a lower risk of GI bleeding, which occurs at a high prevalence in this population (see below), but can we use it? Unfortunately, data to support the use of DOACs remains sparse, and only a handful of case reports and case series have been published describing their use. A 2017 trial with dabigatran vs. warfarin was discontinued prematurely due to the increased risk of thrombotic events in the dabigatran group; however, it is important to recognize the dose of 110mg twice daily is lower than the FDA-approved dose for stroke prevention in atrial fibrillation, which may account for this finding. There was one successful case report and another retrospective case series describing use of apixaban and rivaroxaban for thromboprophylaxis, but longer term data and follow-up are needed to implement any practice changes to the standard use of warfarin in LVADs.
All this has been about clot prevention, but what happens when a clot is detected?
In the setting of an acute thrombosis , there is a paucity of robust data or guideline-based recommendations for clinical management. A variety of surgical, catheter-directed, and pharmacological management strategies are employed.
The ideal approach to management would be urgent device exchange, although this is not feasible in all cases in which pharmacotherapeutic interventions play an important role. As pharmacists, assessing the adequacy of the patient’s current antithrombotic regimen is key to identify whether escalation in anticoagulation intensity or reinitiation of anticoagulation therapy may be warranted.
Currently, no single anticoagulant has been shown to be superior to another for pump thrombosis, but early identification and aggressive treatment with heparin or a direct thrombin inhibitor is a reasonable intervention. These parenteral agents are administered as continuous IV infusions that require meticulous dose titrations based on serial assessments of the aPTT every 4-6 hours until the target aPTT of 1.5-2.5x the baseline is achieved to maximize antithrombotic efficacy and minimize bleeding complications.
It is also reasonable to consider escalating doses of aspirin to 325 mg daily or adding clopidogrel 75 mg daily to the current regimen.
Intravenous glycoprotein IIb/IIIa inhibitors have also been evaluated for pump thrombosis; however, the slight chance of salvaging the device is markedly outweighed by the significantly high rates of bleeding. Eptifibatide at a bolus dose of 180 mcg/kg followed by a continuous infusion at 1 mcg/kg/min for 2 to 4 days demonstrated no clinical improvement in thrombosis in a case series, and all cases evaluated in this review required device exchange. Therefore, the use of GPIIb/IIIa inhibitors should not be advocated for at this time until more promising data, including optimal dosing strategies, is available.
Thrombolytic therapy on the other hand, either intraventricular or systemic, has been studied and determined to be a viable option for LVAD patients who are not surgical candidates. The studies evaluating thrombolysis have employed both IV and intraventricular, with the intraventricular administration benefiting those patients with a higher bleeding risk given the direct delivery into the left ventricle allowing for lower doses to be administered. Patients considered for thrombolytic therapy should be closely evaluated for bleeding, with exclusion criteria similar to those for thrombolytic therapy in acute ischemic stroke.
For a more detailed review on pharmacologic therapy on pump thrombosis, we will refer you to this exceptional review.
LVADs and Bleeding
Paradoxically, although LVADs carry a high thrombotic risk, there is also a complex interplay between hemostatic alterations and device mechanics that predisposes these patients to a high risk of bleeding.
First, a well-established consequence of LVAD mechanics is the development of acquired von-willebrand disease (aVWD) from the shear stress induced by the rapid, continuous blood flow across the device. Rapid blood flow across the device increases vWF degradation, which subsequently impairs platelet aggregation and clot formation.
(Image)
Secondly, with continuous flow devices, the loss of a detectable pulse pressure attenuates perfusion to the GI tract leading to mucosal ischemia and development of arteriovenous malformations (AVM). AVMs are the most frequent causes of severe GI bleeding in LVAD recipients, with an estimated prevalence of 23%, regardless of anticoagulation intensity.
Lastly, these homeostatic alterations are all compounded by the need for routine anticoagulation for thrombophylaxis. Bear in mind that thromboprophylaxis is universal at the time of device implantation, which puts pharmacists in a unique position to optimize the safety of anticoagulation while minimizing any secondary factors that could compound the risk of bleeding.
So now that we know why these patients are prone to bleeds, what do we do when someone does have a bleed?
Initial management of severe bleeding follows the same principles for patients without LVADs and is targeted at restoring intravascular volume with fluids/blood products and repleting VW-factor with desmopressin or cryoprecipitate. It is important to note, judicious use of transfusions should be considered in patients with an LVAD as a bridge to a heart transplant because of the risk of allo-antibody production and sensitization that can complicate transplant compatibility.
A clinical question that may arise is what is the utility of 4-factor prothrombin complex concentrate (4-factor PCC) in the management of LVAD-related bleeding, especially in the setting of warfarin-based anticoagulation?
Historically, the major concern was the elevated risk of thrombosis related to the use of PCC products in LVAD-recipients. However, a 2019 review that analyzed the data surrounding PCC use in patients with active bleeding on continuous flow LVADs demonstrated rapid, predictable reversal with no apparent increased risk of thromboembolism. Compared to FFP, 4F-PCCs are devoid of the time delays, fluid volume, need to cross-type, and transfusion-related reactions that complicate the routine use of FFP. Therefore, use of 4F-PCCs can serve as a viable option in addition to vitamin K for warfarin reversal in patients with CF-LVADs.
PPIs are of no benefit in LVAD-related GI bleeds, but they can be considered while awaiting endoscopic results to determine the location of the bleed. They should be discontinued if no upper GI ulcerations are present.
When you have to figure out how to handle a patient with bleeding AND clotting.
Well. This is awkward. We’ve got a bleeding patient with a device just waiting to thrombose. What now!?
When it comes to managing antithrombotic therapy in acute bleeding, there is no standardized approach. But taking into consideration the clinical picture, the site of bleeding, and the bleeding/thrombotic risk of the patient is essential. Interestingly, GI bleeding has still been documented in patients with therapeutic or sub-therapeutic INRs. This may be explained by the unique pathogenesis of LVAD-related GI bleeding, aVWD and AVMs, and it highlights the need for bleeding treatment and prevention strategies beyond adjustments of anticoagulation to target these hemostatic alterations.
Temporarily withholding antithrombotic treatments is reasonable and may be necessary for patients with major bleeds. In minor bleeds or patients who are hemodynamically stable, reduced anticoagulation intensity by withholding antiplatelets or lowering the INR goal is also a reasonable approach.
In patients with refractory or repeated bleeding episodes, the INR target could be reduced, antiplatelet therapy withdrawal, or all could be discontinued depending on patient-specific factors.
Beyond anticoagulation management, several pharmacologic treatment options have been proposed for the management of LVAD-related GI bleeds.
Octreotide is a somatostatin analogue that has potent vasoconstrictive effects on the splenic vasculature, which results in increased vascular resistance. Octreotide also inhibits angiogenic peptide production that can assist in decreasing formation of angiodysplastic lesions during LVAD support. Octreotide 50 to 100 mcg subcutaneous twice daily or octreotide LAR (long-acting release) 20 to 30 mg intramuscularly (IM) monthly have been used as preventative therapies for patients with chronic or recurrent GI bleeding from AVMs.
Other pharmacologic modalities have been used including thalidomide, estrogen-based hormonal therapy, danazol, and doxycycline. The potential treatments, dosing, and adverse effects are described in the table below. Considering there is no standard guideline to recommend one intervention over another, it is critical for pharmacists to assess each medication based on cost, adherence, and the risk of adverse effects.
One class of agents with proven protective benefits in reducing the recurrence of GI bleeding from AVMs based on recent evidence are ACEi. The proposed mechanism for ACEi is the potent inhibition of RAAS activity and angiotensin-II mediated angiogenesis, thereby reducing the proliferation and formation of malformations.
Lisinopril doses >5mg (or the equivalent) have been shown to reduce the incidence of AVM-related GI bleeding by 57% if initiated within 30 days postoperatively. In reflecting back to the BP management section, the use of ACEi as first-line therapy for patients with LVADs not only provides a mortality benefit for HF, helps to control afterload and BP, but it also can reduce the incidence of AVM formation, resulting in substantial improvement in patient outcomes.
Basically, use an ACEi if you can! The use of ACEi should be strongly advocated for in patients with LVADs in the absence of contraindications due to their multitude of benefits and low risk of harm.
A summary of the management of acute bleeding and secondary prevention of bleeds in LVAD patients.
LVADs and Infections
LVAD-associated infections are leading causes of hospital readmissions and the most common cause of mortality in those who survive >6 months after CF-LVAD implantation. Rates of infections in LVAD are high (~30-50%) and impacted by the type of flow. Pulsatile has a greater incidence of infection compared to continuous flow.
Device implantation carries a risk of infection by introducing bacteria from the surgical devices, which could result in sepsis, endocarditis, bacteremia, or central line infections.
Percutaneous drivelines are exposed to the external environment and without proper maintenance (or if any minor trauma occurs), bacteria can invade and cause superficial driveline-infections or migrate to cause intra-abdominal infections.
Pocket site infections occur when a high inoculum of bacteria accumulates within the pump of the device. These infections are often exceedingly difficult to eradicate because the formation of a biofilm causes a protective barrier around the bacteria inhibiting the penetration of antibiotics. The predominant microorganisms are skin microflora such as Staphylococcus species, e.g., epidermidis or aureus, and Corynebacterium species. Gram negative microorganisms (e.g., Pseudomonas species and Enterobacteriaceae) are more prominent in the immediate postoperative period from hospital exposure or in the later years after LVAD implantation due to exposure of the driveline to the external environment.
(Image)
At the time of device implantation, pharmacists should ensure that patients are receiving appropriate antibiotic prophylaxis. Infection rates in the perioperative period in the absence of prophylaxis are high and can be a major predictor for additional long term infections. In general, cardiothoracic surgery for LVAD implantation is considered a relatively clean procedure.
Despite the increasing rates of multi-drug resistant gram negative or fungal infections, the epidemiology of LVAD infections is largely limited to gram positive skin microflora. The adequate prophylactic regimen for these patients is an area that widely varies in clinical practice. In the pivotal REMATCH trial that led to the widespread use of LVADs for long term management of patients with severe heart failure, the protocol for antibiotic prophylaxis consisted of vancomycin, rifampin, levofloxacin, and fluconazole.
When evaluating the components of this regimen, we are covering every organism from MRSA to Pseudomonas, which seems a bit “overkill” based on the aforementioned etiology of these infections. Taking a deeper dive, we are all fully aware of the ever-growing, considerably severe risks of fluoroquinolones. Given patients are on anticoagulation with warfarin for LVADs, the complex drug-drug interactions with both fluconazole and rifampin can substantially complicate medication management.
In practice, this regimen is used sparingly, if at all.
The ISHLT published guidelines in 2016 for the prophylaxis and treatment of LVAD infections and currently recommend institutions consider using cefazolin or a 3rd or 4th generation cephalosporin with or without vancomycin based on institution and patient-specific risks for MRSA. When verifying orders for peri-operative prophylaxis, timing is key. The infusion should be fully administered 30 minutes to 1 hour prior to incision to ensure adequate serum concentrations at the time of operation until full chest closure.
Now for vancomycin, given the long infusion times, this means patients may need to start as early as 2 hours prior to surgery. If there is significant blood loss during the procedure (>1,500 ml) or large volumes (2 units of packed red blood cells) are given, antibiotics should be re-dosed to maintain adequate tissue concentrations.
Antibiotics are only indicated for 48 hours after surgery, and as antibiotic stewards, we must be cognizant of this firm recommendation given there is no benefit (but certainly higher rates of resistance) associated with longer durations.
An important focus after device implantation is educating about infection prevention strategies such as daily cleansing of drivelines, washing with chlorhexidine, and minimizing any trauma to the exit site. Long term preventative antibiotics are not supported by robust literature and can lead to resistance or increased rates of Clostridium difficile. However, some clinicians may elect to use prophylaxis if they foresee that definitive management with device exchange would not be feasible if infection occurs.
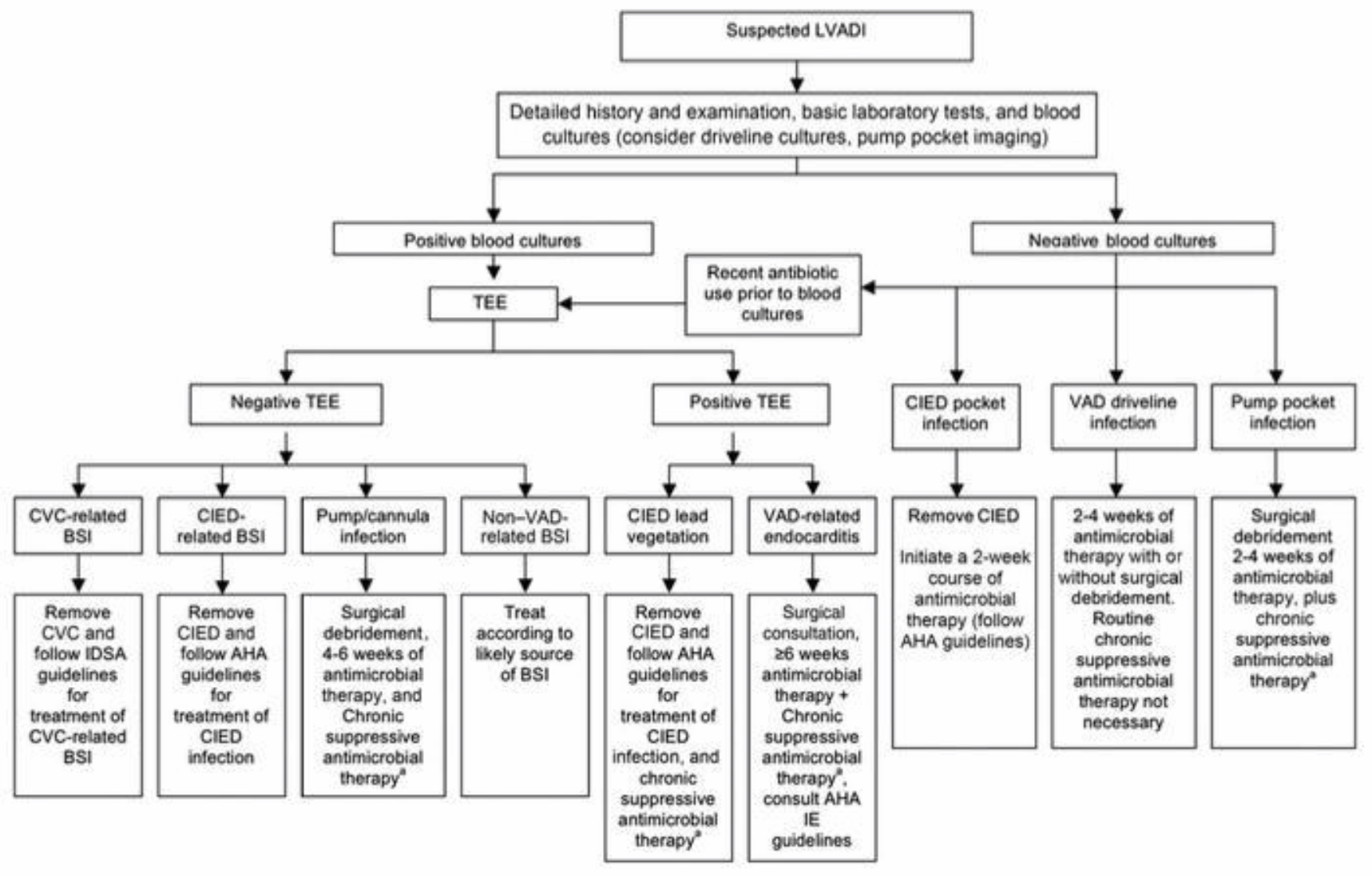
Given the sequelae of complications that can occur from LVAD infections, prompt identification and management are imperative to maintain device patency and prevent septic complications. Treatment is cumbersome given the ISHLT guidelines do not provide a specific recommendation on the selection of antibiotics or duration of therapy.
In uncomplicated driveline infections (no bacteremia, hemodynamic compromise, invasive infections), oral treatment targeting skin microflora, such as doxycycline, trimethoprim-sulfamethoxazole, or oral beta-lactams, are reasonable choices. In patients with diabetes or recent water exposure, empiric use of an oral fluoroquinolone for Pseudomonas coverage can be added. In patients with invasive infections, broad spectrum IV treatment is warranted and should be targeted towards the suspected pathogens with de-escalation based on culture results.
Patients with deep seeded pocket infections or gram positive bloodstream infections may require up to 6 weeks of therapy. From a pharmacists perspective, ensuring antibiotic adherence and using the most narrow spectrum agent is essential, as well as monitoring warfarin therapy due to the impact antimicrobials have on the INR.
Interestingly, LVAD infections can actually potentiate the activation of platelets and the clotting cascade, which compounds the risk of thrombotic complications in LVAD patients. Some experts consider intensification of antiplatelet or anticoagulant treatment during acute episodes of infection to prevent from severe thrombotic sequelae, making anticoagulation monitoring even more crucial during treatment.
A summary of LVAD infection management. Don’t forget to check out the FREE tl;dr antibiotic cheat sheet if you haven’t already!
LVADs and Cardiac Arrest
The approach to managing a cardiac arrest in an LVAD patient can be a rather challenging and unique situation. As previously mentioned, a pulse may not be detected with continuous-flow LVADs, making physical assessment a significant challenge. A comprehensive assessment of the LVAD, including placement, device parameters, and alarms, should be performed to help determine if the etiology of arrest was secondary to device failure or an alternative cause.
The approach to evaluating a patient with an LVAD should begin with ensuring the device is functioning. Placing your ear up to the patients chest and listening for a humming sound is reflective of the operating rotors in the device and is indicative of appropriate device mechanics. If the humming sound is not detected, it can be assumed that the device is malfunctioning and providers should proceed with standardized ACLS protocols.
Although considered a lifesaving intervention, for years emergency response teams withheld from chest compressions in the crashing LVAD patient due to the theorized risk of damaging or dislodging the device. This theory has yet to hold true in clinical practice and in fact, has shown to have detrimental outcomes in these individuals not treated with standard of care.
Thus, the AHA/ACC developed a consensus statement that emphasizes the standardized ACLS algorithm, including chest compressions, should be performed in all patients with an LVAD. Now the question becomes, how do we assess the efficacy of our resuscitative efforts if we are unable to detect a pulse with continuous-flow devices?
Utilizing waveform capnography is the single most important marker of adequate CPR interventions and should be used to guide resuscitation efforts. Pharmacists (yes you!) are integral members of code blue response teams and we can make a significant impact on patient outcomes by advocating for early interventions such as CPR and facilitate medication administration to improve the chances that ROSC is achieved.
Conclusion
The development and implementation of LVADs have provided options for patients with end-stage HF. In turn, the use of these devices has been on the rise and will continue to increase. It is critical for pharmacists to have an awareness of the mechanics of these devices and related pharmacotherapy issues in order to help gauge hemodynamics and drive treatment decisions.
Pharmacists should be aware of appropriate doppler blood pressure measurement in order to assess the need or changes to anti-hypertensive/heart failure therapy. It is also critical for pharmacists to understand the fine balance between the need for anticoagulation for the prevention of device thrombosis, changes to coagulation parameters due to the device, and the prevention and management of bleeding.
In addition, pharmacists who do not routinely care for these patients should seek consultation with pharmacists or other providers who have experience with these patients to assure optimal care management. Use your resources!!
Just a quick reminder…if you’d like a downloadable (and printer-friendly) version of this article, you can get one here.